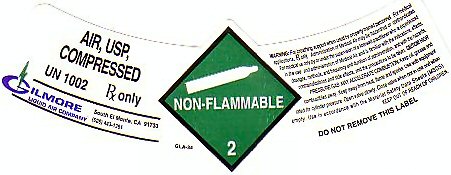
AIR, USP COMPRESSED LABEL
AIR, USP, COMPRESSED UN 1002 NON-FLAMMABLE-2 Rx ONLY
WARNING: FOR BREATHING SUPPORT WHEN USED BY PROPERLY TRAINED PERSONNEL. FOR MEDICAL APPLICATIONS Rx ONLY. ADMINISTRATION OF MEDICAL AIR MAY BE HAZARDOUS OR CONTRAINDICATED. FOR MEDICAL USE ONLY BY OR UNDER THE SUPERVISION OF A LICENSED PRACTITIONER WHO IS EXPERIENCED IN THE USE AND ADMINISTRATION OF MEDICAL AIR AND IS FAMILIAR WITH THE INDICATIONS, EFFECTS, DOSAGES, METHODS, AND FREQUENCY AND DURATION OF ADMINISTRATION, AND WITH THE HAZARDS AND PRECAUTIONS TO BE TAKEN. CAUTION: HIGH PRESSURE GAS MAY ACCELERATE COMBUSTION. KEEP OIL GREASE AND COMBUSTIBLES AWAY. KEEP AWAY FROM HEAT, FLAME AND SPARKS. USE WITH EQUIPMENT RATED FOR CYLINDER PRESSURE. OPEN VALVE SLOWLY. CLOSE VALVE WHEN NOT IN USE AND WHEN EMPTY. USE IN ACCORDANCE WITH THE MATERIAL SAFETY DATA SHEET (MSDS) KEEP OUT OF REACH OF CHILDREN. DO NOT REMOVE THIS PRODUCT LABEL.