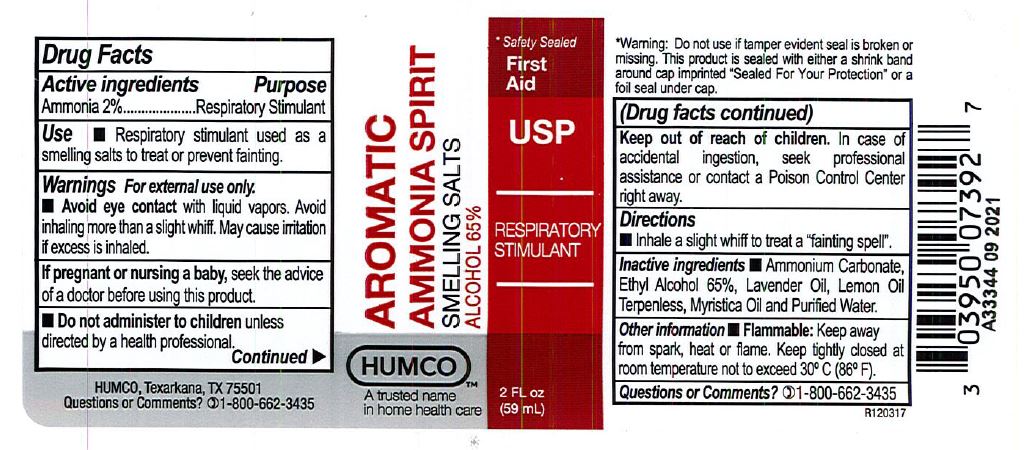
Active Ingredient
Ammonia 2 %
Purpose
Respiratory Stimulant
Use
Respiratory stimulant used as a smelling salts to treat or prevent fainting.
Warnings
For external use only. Avoid eye contact with liquid vapors. Avoid inhaling more than a slight whiff. May cause irritation if excess is inhaled.
If pregnant or nursing a baby
seek the advice of a doctor before using this product.
Keep out of the reach of children
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center right away.
Directions
Inhale a slight whiff to treat a "fainting spell"
Inactive Ingredients
Ammonium Carbonate, Ethyl Alcohol 65%, Lavender Oil, Lemon Oil Terpenless, Myristica Oil and Purified Water.
Other Information
Flammable: Keep away from spark, heat or flame. Keep tightly closed at room temperature not to exceed 30C (86F).
Questions or Comments?
1-800-662-3435
Label
Humco Holding Group, Inc.
