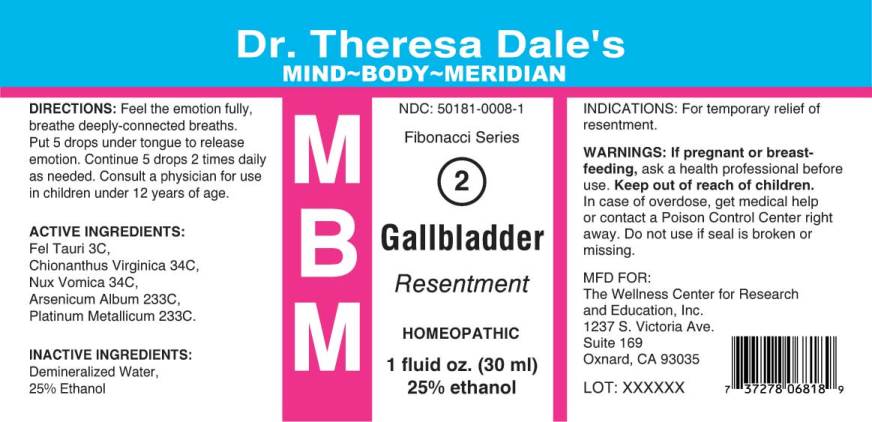
MBM 2 GALLBLADDER- fel tauri, chionanthus virginica, nux vomica, arsenicum album, plantinum metallicum liquid
The Wellness Center
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS:
Fel Tauri 3C, Chionanthus Virginica 34C, Nux Vomica 34C, Arsenicum Album 233C, Plantinum Metallicum 233C.
INDICATIONS:
For temporary relief of resentment.
WARNINGS:
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Do not use if seal is broken or missing.
DIRECTIONS:
Feel the emotion fully, breath deeply-connected breaths. Put 5 drops under tongue to release emotion. Continue 5 drops 2 times daily as needed. Consult a physician for use in children under 12 years of age.
INACTIVE INGREDIENTS:
Demineralized Water, 25% Ethanol.
KEEP OUT OF REACH OF CHILDREN:
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Do not use if seal is broken or missing.
INDICATIONS:
For temporary relief of resentment.
QUESTIONS:
MFD. FOR:
The Wellness Center for Research
and Education, Inc.
1237 S. Victoria Ave.
Suite 169
Oxnard, CA 93035
PACKAGE LABEL DISPLAY:
Dr. Theresa Dale's
Mind~Body~Meridian
MBM
NDC: 50181-0008-1
Fibonacci Series
2
Gallbladder
Resentment
HOMEOPATHIC
1 fluid oz. (30 ml)