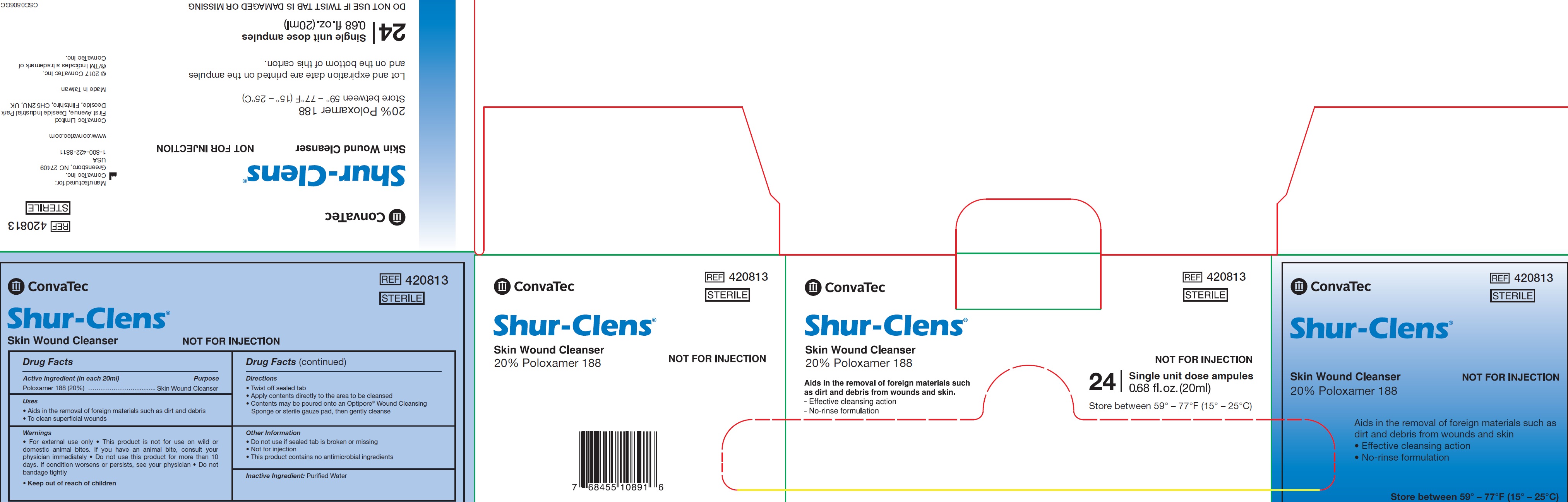
Uses
• Aids in the removal of foreign materials such as dirt and debris
• To clean superficial wounds
Warnings
• For external use only • This product is not for use on wild or domestic animal bites. If you have an animal bite, consult your physician immediately • Do not use this product for more than 10 days. If condition worsens or persists, see your physician • Do not bandage tightly
Directions
• Twist off sealed tab
• Apply contents directly to the area to be cleansed
• Contents may be poured onto an Optipore® Wound Cleansing Sponge or sterile gauze pad, then gently cleanse