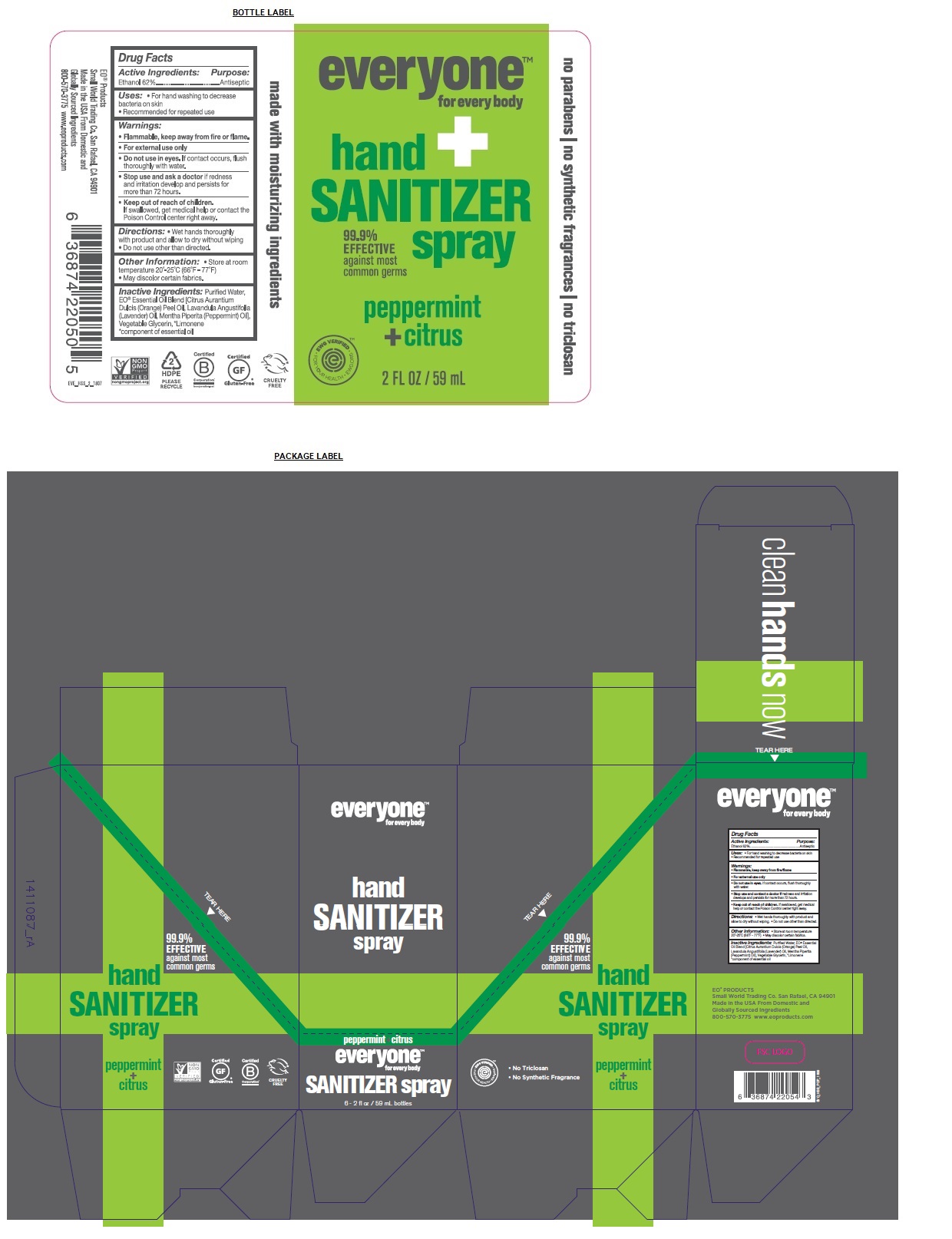
EVERYONE HAND SANITIZER PEPPERMINT CITRUS- alcohol spray
EO Products, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredients:
Ethanol 62%
Uses:
- For hand washing to decrease bacteria on skin
- Recommended for repeated use
Warnings:
-
Flammable, keep away from fire/flame
-
For external use only
-
Do not use in eyes. If contact occurs, flush thoroughly with water.
-
Stop use and contact a doctor if redness and irritation develops and persists for more than 72 hours.
-
Keep out of reach of children. If swallowed, get medical help or contact the Poison Control center right away.
Directions:
- Wet hands thoroughly with product and allow to dry without wiping.
- Do not use other than directed.
Inactive Ingredients:
Purified Water, EO® Essential Oil Blend [Citrus Aurantium Dulcis (Orange) Peel Oil, Lavandula Angustifolia (Lavender) Oil, Mentha Piperita (Peppermint) Oil], Vegetable Glycerin, * Limonene
* component of essential oil
Other Information:
- Store at room temperature 20°-25°C (66°F - 77°F)
- May discolor certain fabrics
99.9% EFFECTIVE against most common germs
clean hands now
no parabens / no synthetic fragrances / no triclosan
made with moisturizing ingredients
EO® PRODUCTS
Small World Trading Co. San Rafael, CA 94901
Made in the USA From Domestic and Globally Sourced Ingredients
800-570-3775 www.eoproducts.com
Packaging