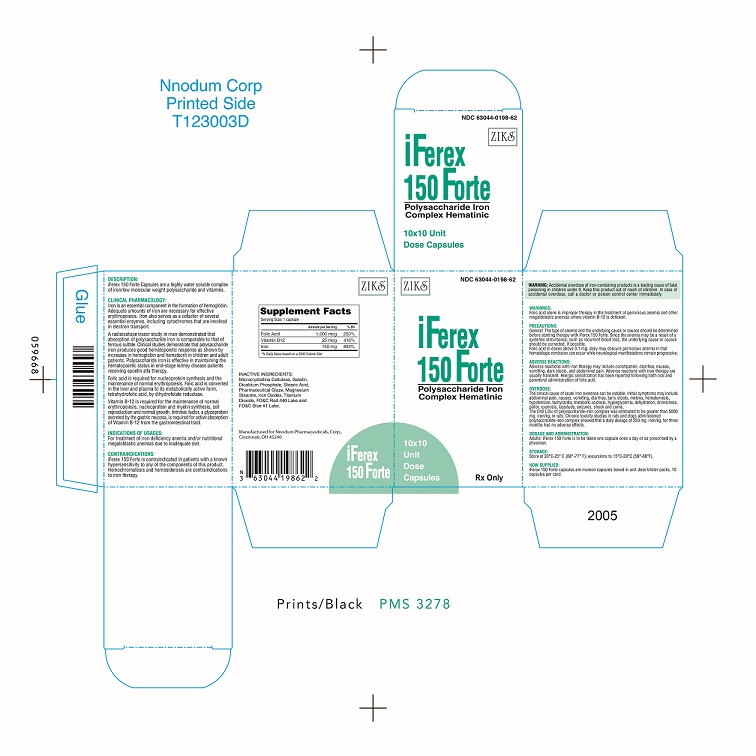
WARNINGS:
Folic acid alone is improper therapy fin the treatment of pernicious anemia and other megaloblastic anemias where vitamin B-12 is deficient.
PRECAUTIONS:
Folic acid in doses above 0..1 mg - 0..4 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations remain progressive.
Pediatric Use Safety and effectiveness in pediatric patients has not been established.
SAFE HANDLING WARNING:
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6.
Keep this products out of the reach of children. In case of accidental overdose, call a doctor or poison control immediately.