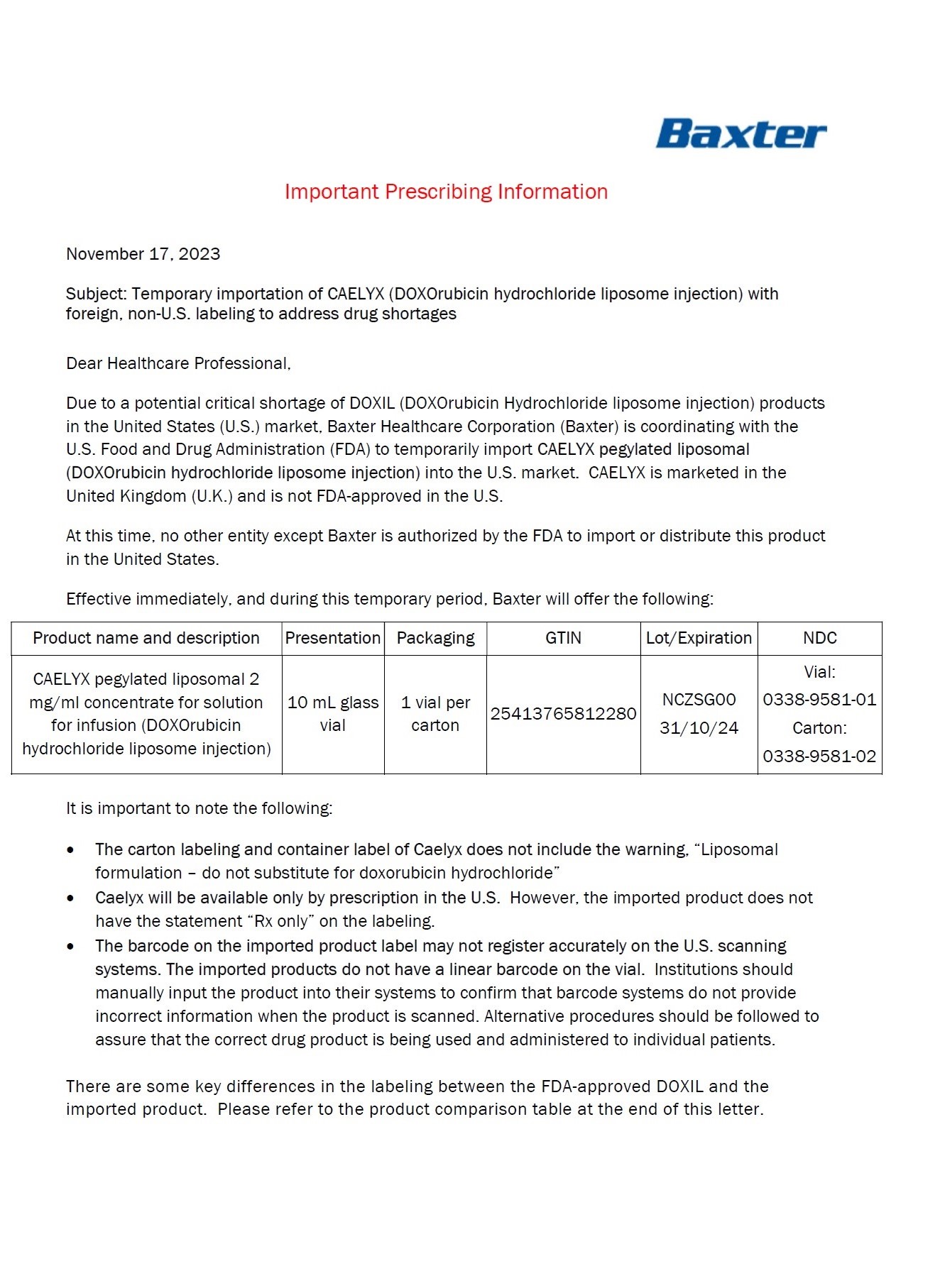
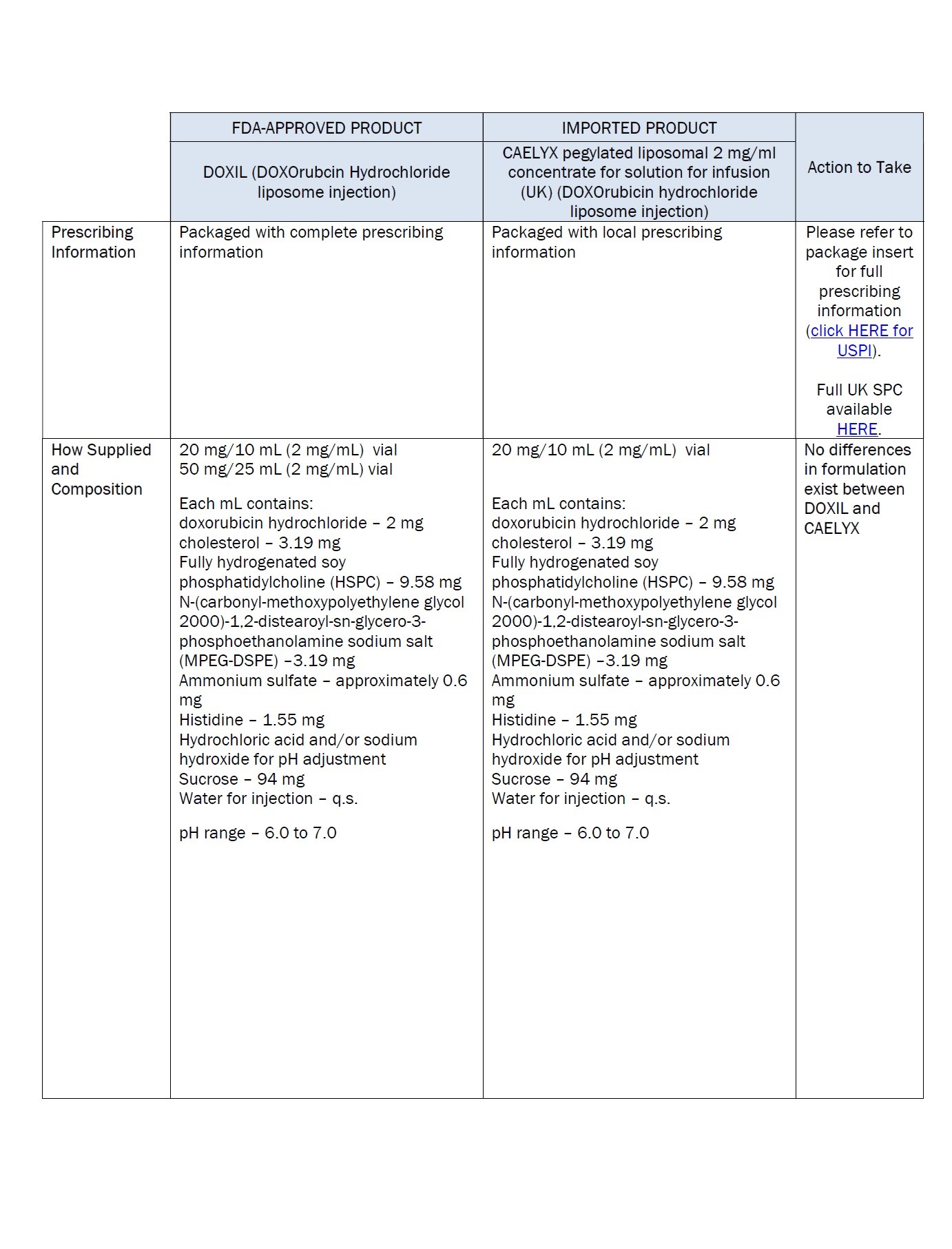
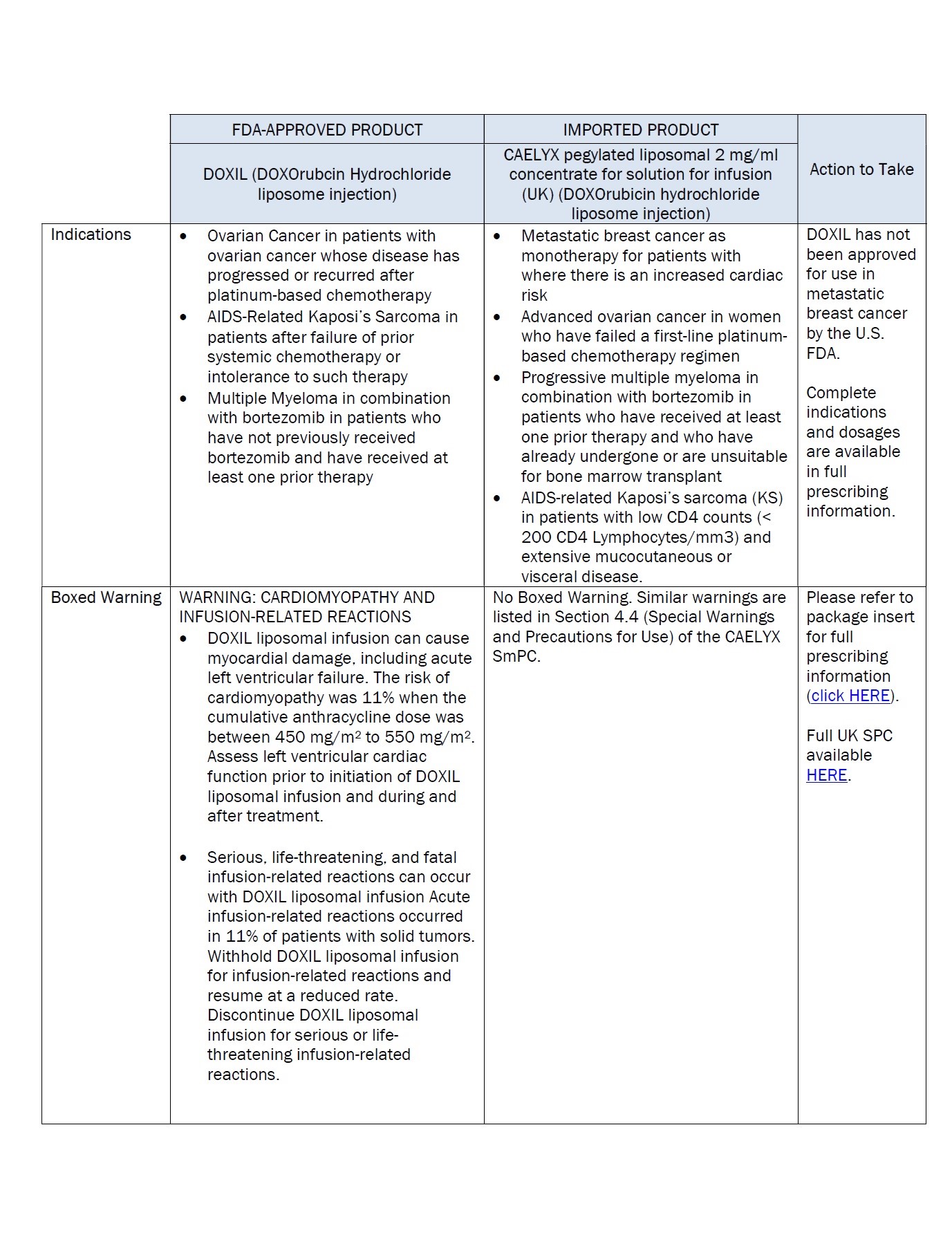
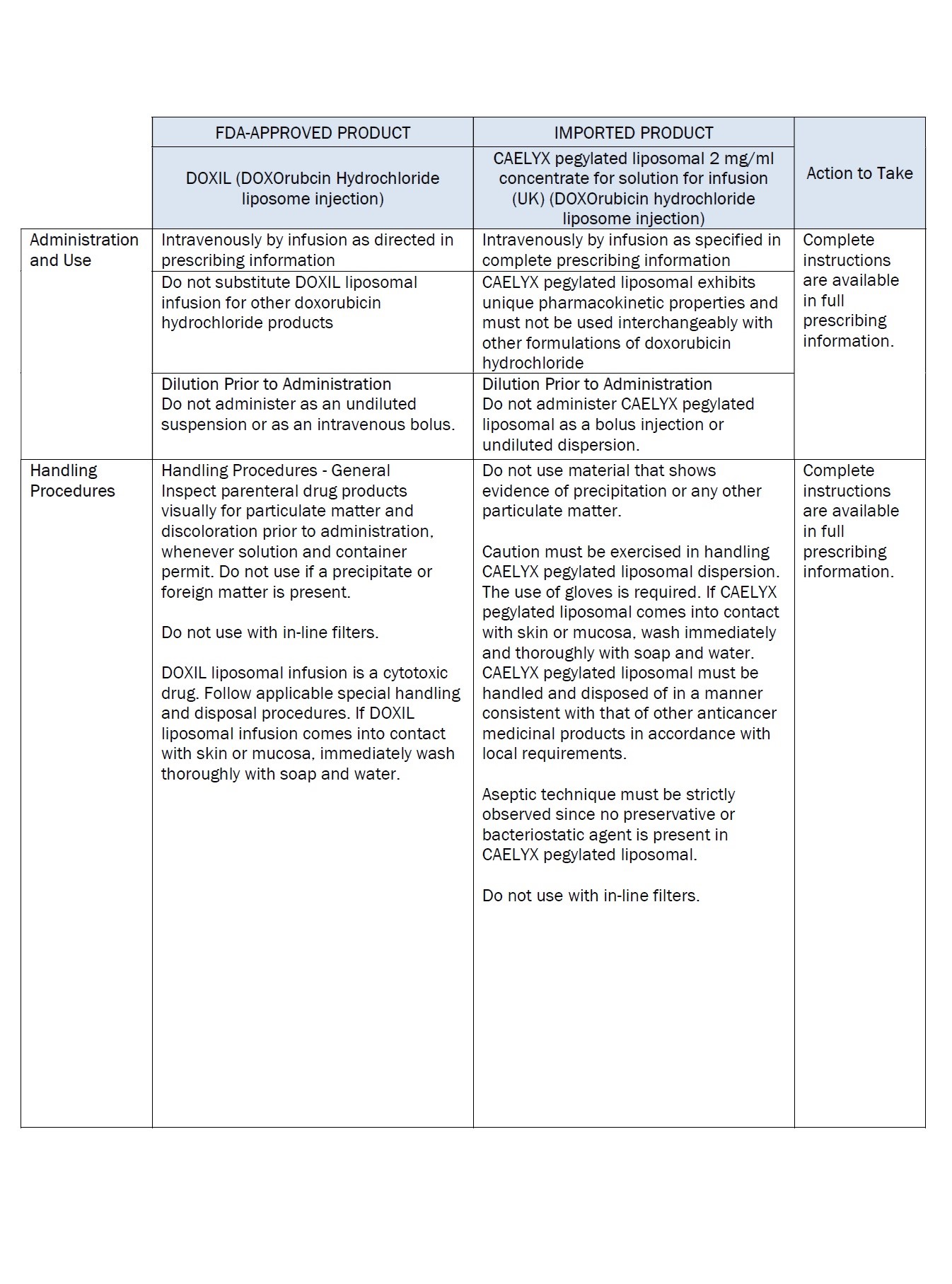
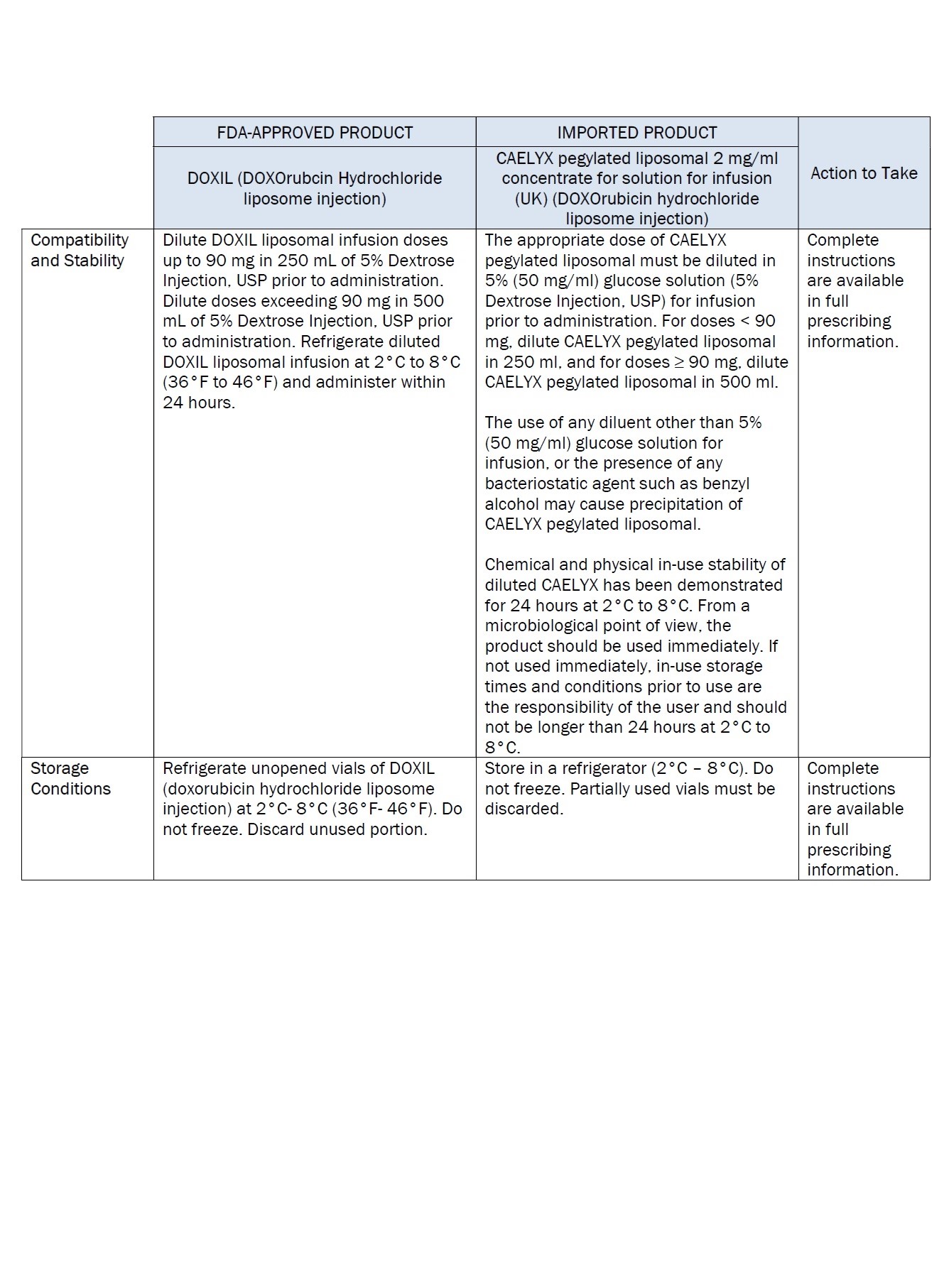
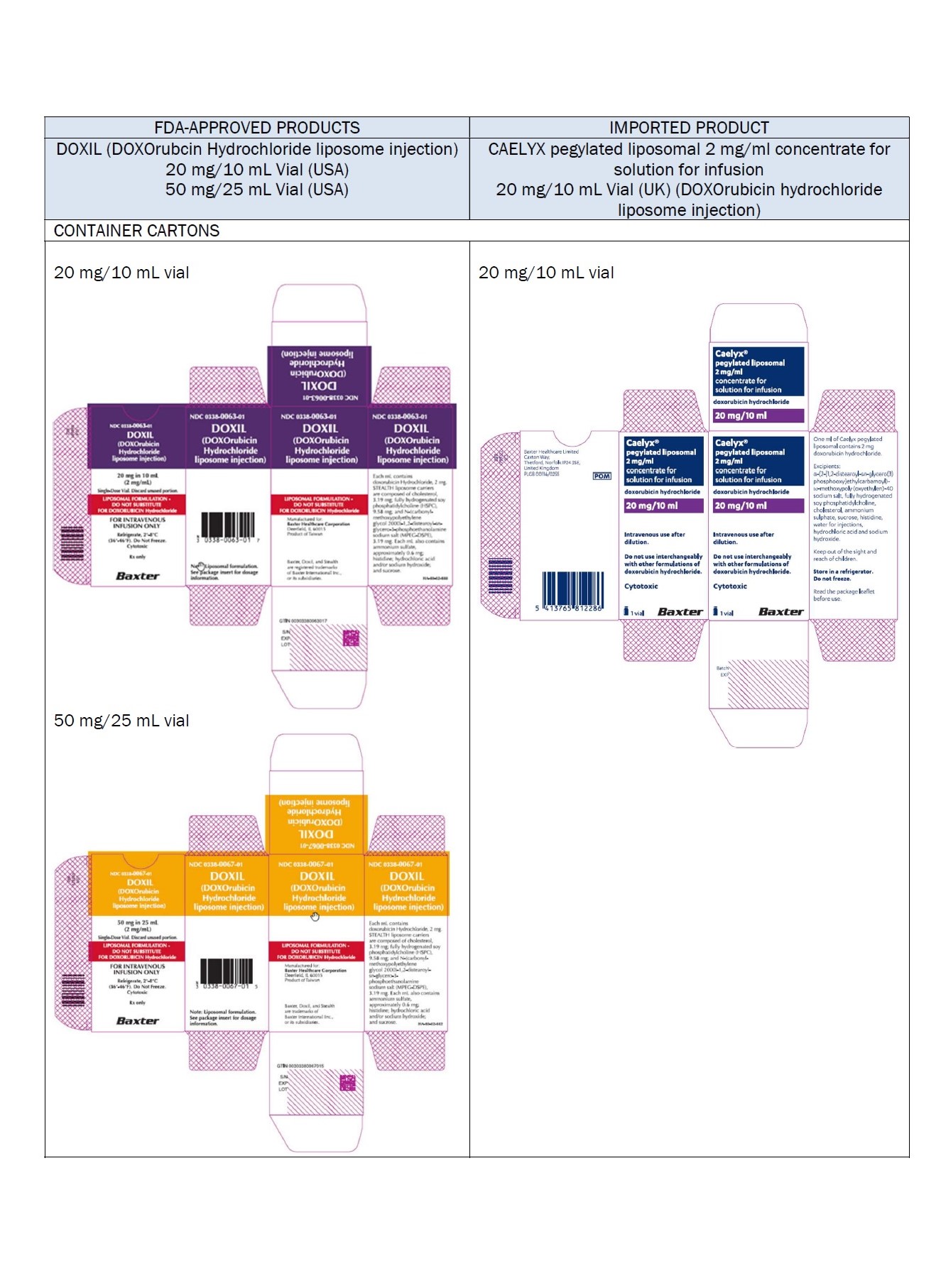
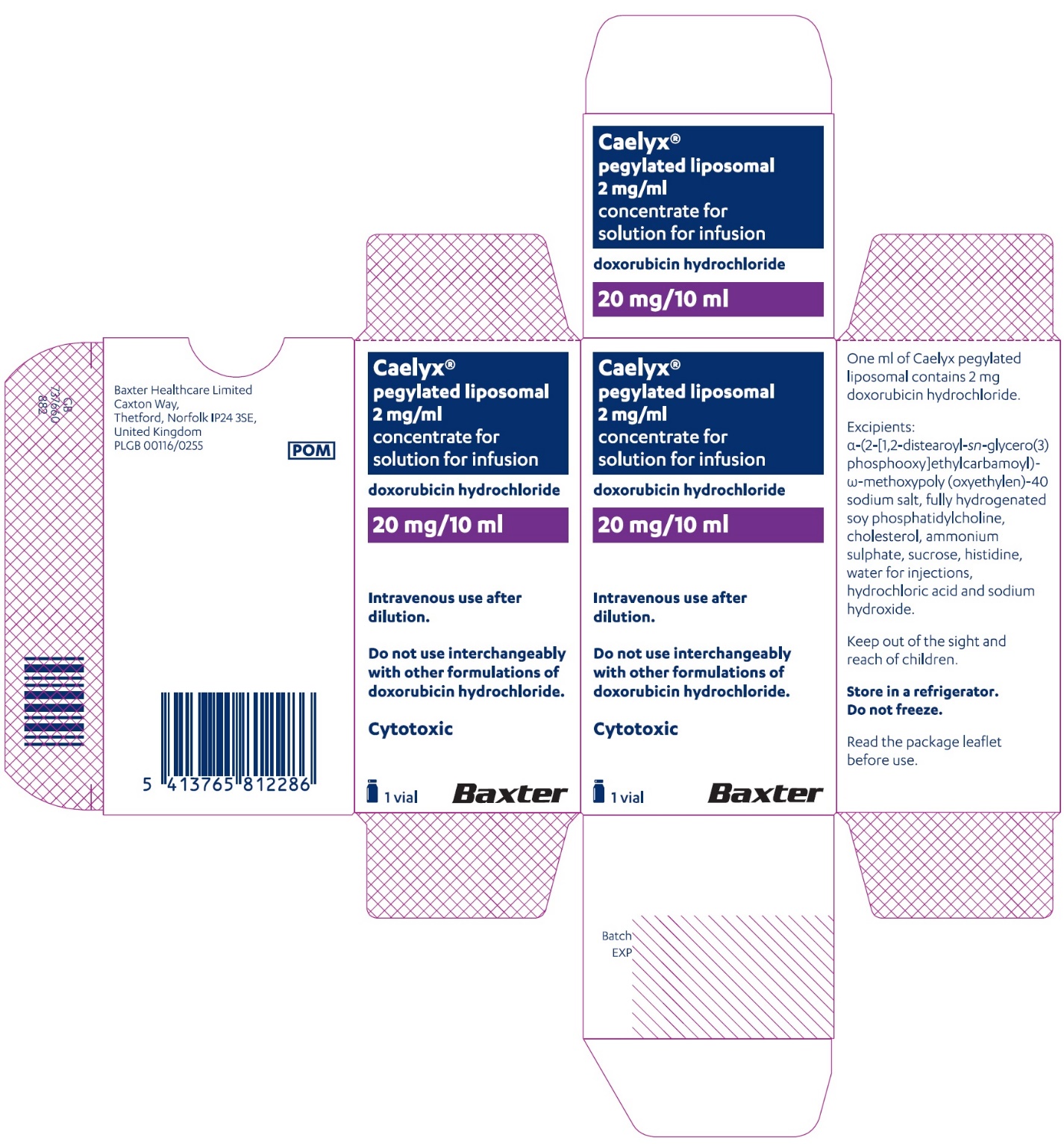
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 20 mg/10 mL
Caelyx® pegylated liposomal
2 mg/ml
sterile concentrate
doxorubicin hydrochloride
20 mg/10 mL
IV after dilution.
Caelyx®
pegylated liposomal
2 mg/ml
concentrate for
solution for infusion
doxorubicin hydrochloride
20 mg/10 mL
Intravenous use after
dilution.
Do not use interchangeably
with other formulations of
doxorubicin hydrochloride.
Cytotoxic
1 vial
Baxter Logo
One ml of Caelyx pegylated
liposomal contains 2 mg
doxorubicin hydrochloride.
Excipients: α-(2-[1,2-distearoyl-sn-glycero(3)
phosphooxy]ethylcarbamoyl)
-ϖ-methoxypoly(oxyethylen)-40
sodium salt, fully hydrogenated
soy phosphatidylcholine,
cholesterol, ammonium
sulphate, sucrose, histidine,
water for injections,
hydrochloric acid and sodium
hydroxide.
Keep out of the sight and
reach of children.
Store in a refrigerator.
Do not freeze.
Read the package leaflet
before use.
Baxter Healthcare Limited
Caxton Way,
Thetford,
Norfolk,
IP24 3SE,
United Kingdom
PLGB 00116/0255