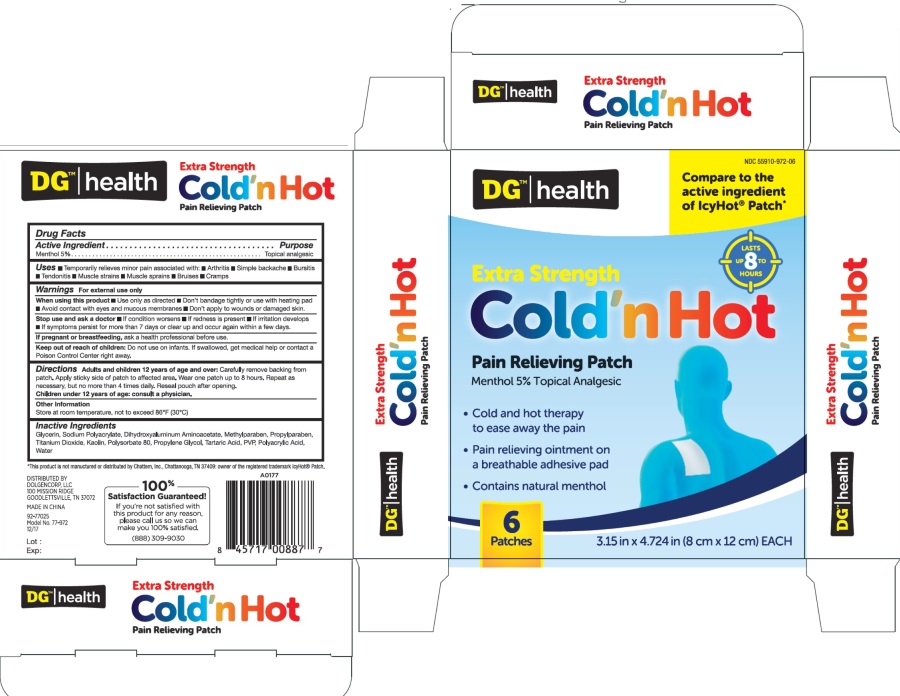
ACTIVE INGREDIENT
Active Ingredient ...............................................................................................Purpose
Menthol 5%...............................................................................................Topical Analgesic
INACTIVE INGREDIENT
Glycerol, Sodium Polyacrylate, Water, Dihydroxyaluminum Aminoacetate, Methylparaben, Propylparaben, Titanium
Dioxide, Kaolin, Tween 80, Propylene Glycol, Span 80, Tartaric Acid, PVP, Polyacrylic Acid
INDICATIONS & USAGE
Temporarily relieves minor pain associated with: ■ arthritis ■ simple backache ■ bursitis ■ tendonitis
■ muscle strains ■ muscle sprains ■ bruises ■ cramps
DOSAGE & ADMINISTRATION
Adults and children over 12 years: Carefully remove backing from patch. Apply sticky side of patch to affected area.
Wear one patch up to 8 hours. Repeat as necessary, but no more than 4 times daily. Reseal pouch after opening.
Children 12 years or younger: Consult a physician.
When using this product
Use only as directed ■ Don’t bandage tightly or use with heating pad
■ Avoid contact with eyes and mucous membranes ■ Don’t apply to wounds or damaged skin.