HEALTHY ACCENTS HEMORRHOIDAL- mineral oil, petrolatum, phenylephrine hcl ointment
DZA Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DZA Brands, LLC Hemorrhoidal Ointment Drug Facts
Uses
- •
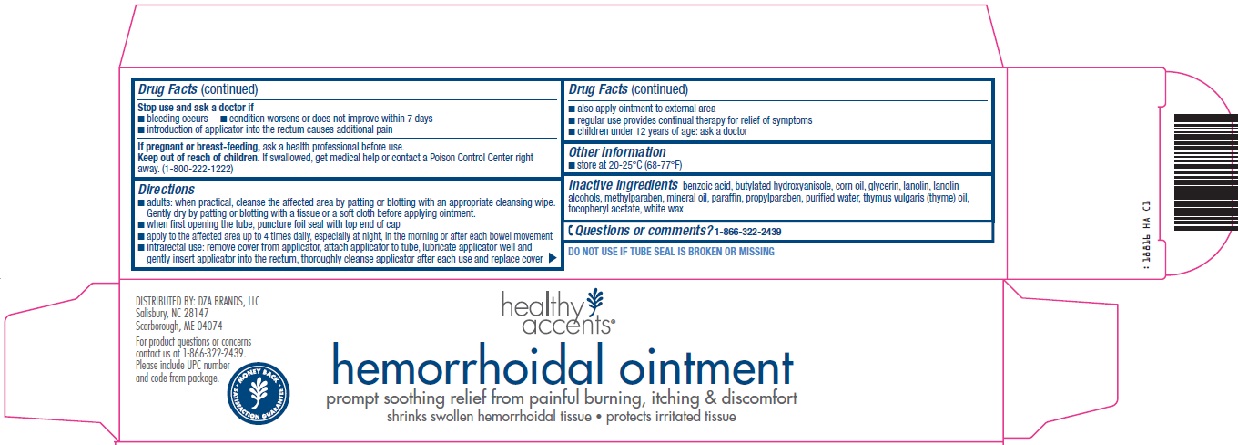
- helps relieve the local itching and discomfort associated with hemorrhoids
- •
- temporarily shrinks hemorrhoidal tissue and relieves burning
- •
- temporarily provides a coating for relief of anorectal discomforts
- •
- temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
Warnings
For external and/or intrarectal use only
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression.
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
- •
- adults: when practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or a soft cloth before applying ointment.
- •
- when first opening the tube, puncture foil seal with top end of cap
- •
- apply to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement
- •
- intrarectal use: remove cover from applicator, attach applicator to tube, lubricate applicator well and gently insert applicator into the rectum, thoroughly cleanse applicator after each use and replace cover
- •
- also apply ointment to external area
- •
- regular use provides continual therapy for relief of symptoms
- •
- children under 12 years of age: ask a doctor
Inactive ingredients
benzoic acid, butylated hydroxyanisole, corn oil, glycerin, lanolin, lanolin alcohols, methylparaben, mineral oil, paraffin, propylparaben, purified water, thymus vulgaris (thyme) oil, tocopheryl acetate, white wax
Package/Label Principal Display Panel
Compare to Preparation H® Hemorrhoidal Ointment active ingredients
hemorrhoidal ointment
prevents further irritation
prompt soothing relief from painful burning, itching & discomfort
shrinks swollen hemorrhoidal tissue – protects irritated tissue
relieves internal and external discomfort
NET WT 2 OZ (57g)
| HEALTHY ACCENTS HEMORRHOIDAL
mineral oil, petrolatum, phenylephrine hcl ointment |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - DZA Brands LLC (090322194) |