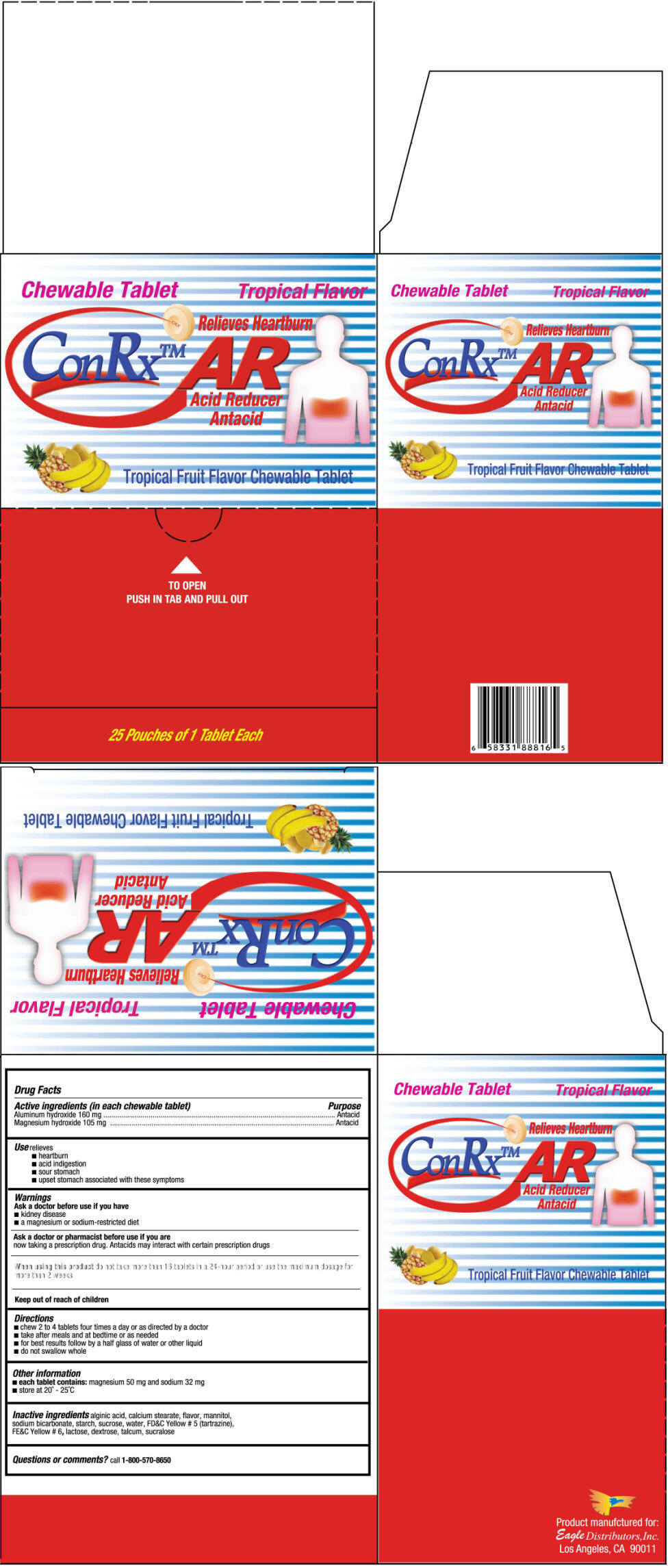
CONRX AR- aluminum hydroxide and magnesium hydroxide tablet, chewable
Eagle Distributors,Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients (in each chewable tablet) | Purpose |
| Aluminum hydroxide 160 mg | Antacid |
| Magnesium hydroxide 105 mg | Antacid |
Use
- heartburn
- acid indiestion
- sour stomach
- upset stomch associated with these symptoms
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium or sodium-restricted diet
Ask a doctor or pharmacist before use if you are
now taking a prescription durg. Antacids may interact with certain prescription drugs
When using this product do not take more than 16 tablets in a 24-hour period or use the Maximum dosage for more than 2 wekks
Keep out of reach of children.
Directions
- chew 2 to 4 tablets four times a day or as directed by a doctor
- take after meals and at bedtime or as needed
- for best results follow by a half glass of water or other liquid
- do not swallow whole
Other information
-
each tablet contains: magnesium 50 mg and sodium 32 mg
- store at 20°-25°C
Inactive ingredients
alginic acid, calcium stearate, flavor, mannitol, sodium bicarbonate, starch, sucrose, water, FD&C Yellow # 5 (tartrazine), FE&C Yellow # 6, lactose, dextrose, talcum, sucralose
Questions or comments?
1-800-570-8650
PRINCIPAL DISPLAY PANEL - 50 Pouch Box
Chewable Tablet
Tropical Flavor
Relieves Heartburn
ConRx™ AR
Acid Reducer
Antacid
Tropical Fruit Flavor Chewable Tablet
TO OPEN
PUSH IN TAB AND PULL OUT
25 Pouches of 1 Tablet Each
Eagle Distributors,Inc.