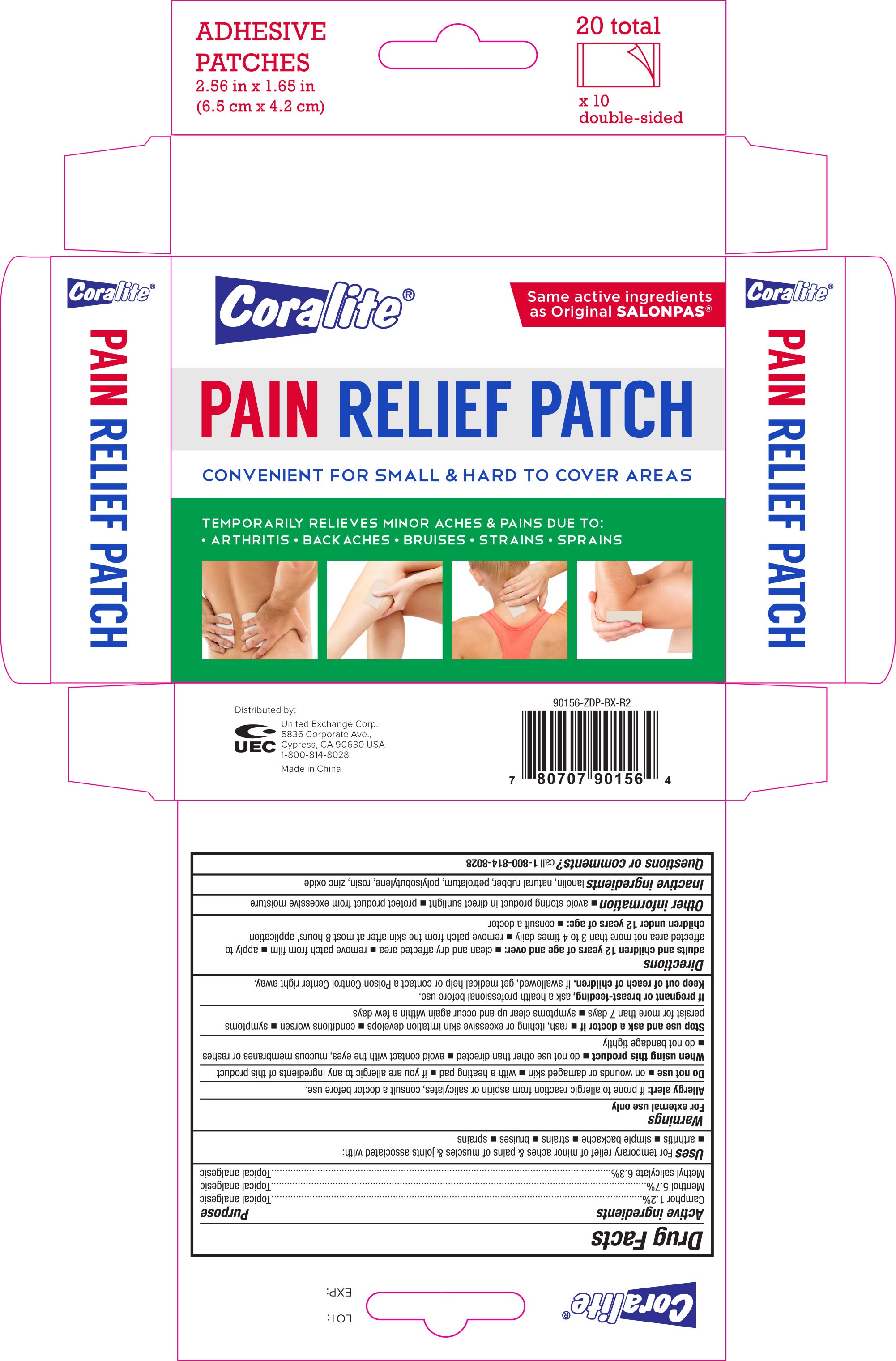
CORALITE PAIN RELIEF- dl-camphor, l-menthol, methylsalicylate patch
United Exchange Corp.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Coralite Pain Patch 40 ct. 90156 (2018)
Coralite Pain Relief Patch 20 Ct
Active ingredients Purpose
DL-Camphor 1.2%.................................. Topical Analgesic
L-Menthol 5.7%...................................... Topical Analgesic
Methyl Salicylate 6.3%............................ Topical Analgesic
Uses
For temporary relief of minor aches and pains of muscles and joints associated with:
- arthritis
- simple backache
- strains
- bruises
- sprains
Do not use
- on wounds or damaged skin
- if you are allergic to aspirin or salicylates
- with a heating pad
- with, or at the same time as, other external analgesic products
When using this product
- do not use other than directed
- avoid contact with the eyes, mucous membranes or rashes
Stop use and ask a doctor if
- rash, itching or excessive skin irritation develops
- conditions worsen symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
Keep out of reach of children. If swallowed, get medical help or conduct a Poision Control Center right away
Caution: This product contains natural rubber latex which may cause allergic reactions
| CORALITE PAIN RELIEF
dl-camphor, l-menthol, methylsalicylate patch |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - United Exchange Corp. (840130579) |