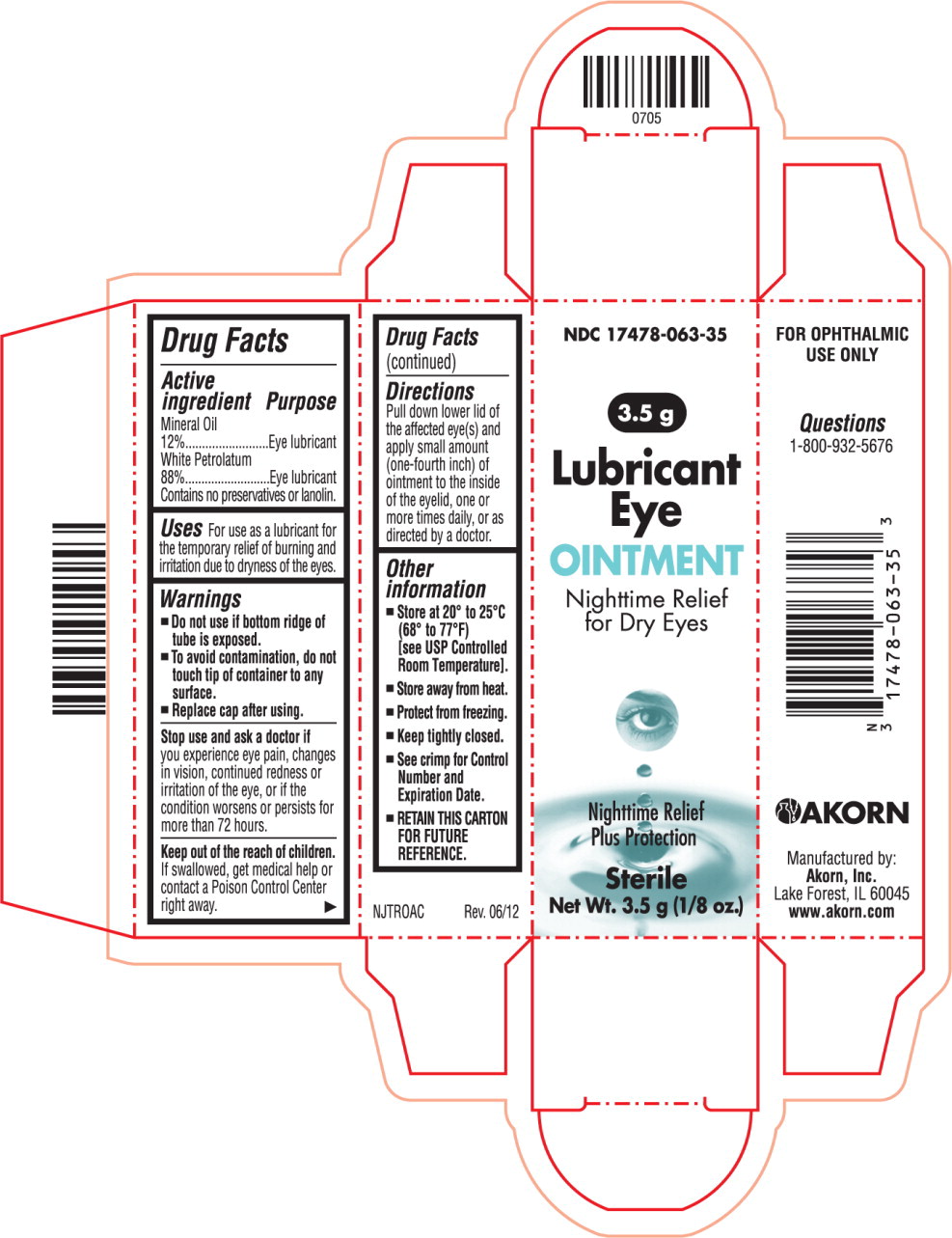
LUBRICANT EYE- mineral oil and petrolatum ointment
Akorn
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Mineral Oil
12%
White Petrolatum
88%
Contains no preservatives or lanolin.
Purpose
Eye lubricant
Eye lubricant
Uses
For use as a lubricant for the temporary relief of burning and irritation due to dryness of the eyes.
Warnings
-
Do not use if bottom ridge of tube is exposed.
-
To avoid contamination, do not touch tip of container to any surface.
-
Replace cap after using.
Stop use and ask a doctor if you experience eye pain, changes in vision, continued redness or irritation of the eye, or if the condition worsens or persists for more than 72 hours.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
Pull down lower lid of the affected eye(s) and apply small amount (one-fourth inch) of ointment to the inside of the eyelid, one or more times daily, or as directed by a doctor.
Other information
-
Store at 20° to 25°C (68° to 77°F)
[see USP Controlled Room Temperature].
-
Store away from heat.
-
Protect from freezing.
-
Keep tightly closed.
-
See crimp for Control Number and Expiration Date.
-
RETAIN THIS CARTON FOR FUTURE REFERENCE.
NJTROAC Rev. 06/12
Principal Display Panel Text for Container Label:
NDC 17478-063-35 Akorn Logo
Lubricant Eye OINTMENT
Nighttime Relief For Dry Eyes Sterile
FOR OPHTHALMIC USE ONLY Net Wt. 3.5 g (1/8oz.)
Principal Display Panel Text for Carton Label:
NDC 17478-063-35
3.5 g
Lubricant
Eye
OINTMENT
Nighttime Relief
For Dry Eyes
Nighttime Relief
Plus Protection
Sterile
Net Wt. 3.5 g (1/8oz.)