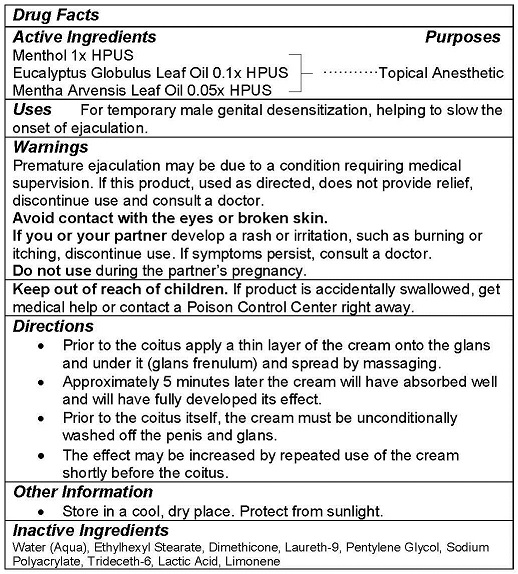
ACTIVE INGREDIENTS
Menthol 1x HPUS
Eucalyptus Globulus Leaf Oil 0.1X HPUS
Mentha Arvenses Leaf Oil 0.05x HPUS
WARNINGS
Premature ejaculation may be due to a condition requiring medical supervision. If this product, used as directed, does not provide relief, discontinue use and consult a doctor.
Avoid contact with the eyes or broken skin.
If you or your partner develop a rash or irritation, such as burning or itching, discontinue use. If symptoms persist, consult a doctor.
Do not use during the partner’s pregnancy.
Keep out of reach of children. If product is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
- Prior to the coitus apply a thin layer of the cream onto the glans and under it (glans frenulum) and spread by massaging.
- Approximately 5 minutes later the cream will have absorbed well and will have fully developed its effect.
- Prior to the coitus itself, the cream must be unconditionally washed off the penis and glans.
- The effect may be increased by repeated use of the cream shortly before the coitus.