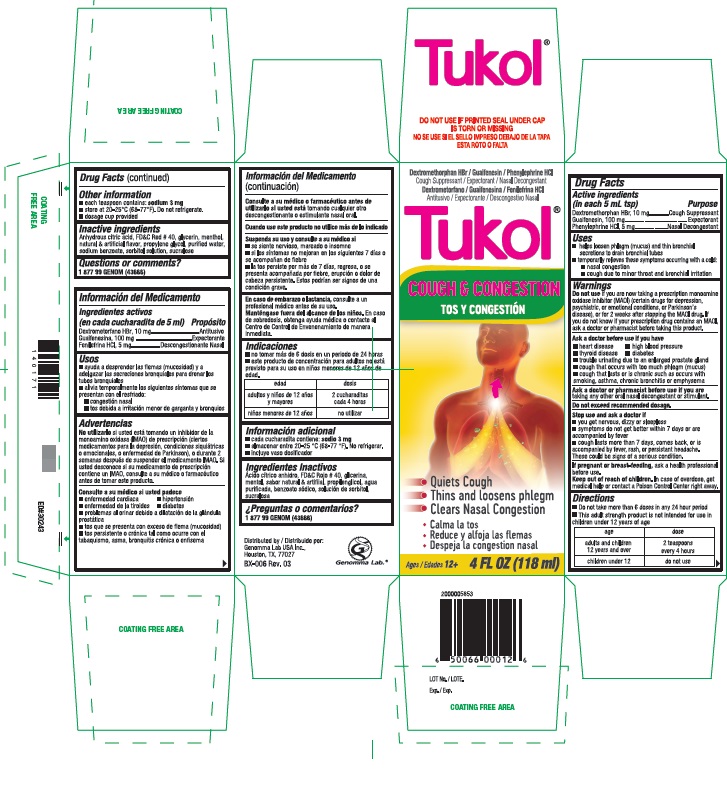
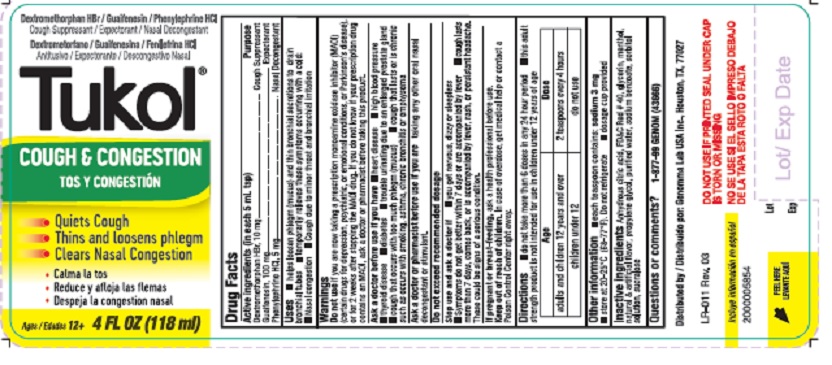
Active ingredients
DRUG FACTS
Active ingredients
(in each 5 mL tsp)
Dextromethorphan HBr, USP 10 mg
Guaifenesin, USP 100 mg
Phenylephrine HCL, USP 5 mg
Keep out of reach of children
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Uses
- help loosen phlegm (mucus) and thin bronchial secretions to drain bronchial tubes
- temporarily relieves these symptoms occurring with a cold
- nasal congestion
- cough due to minor throat and bronchial irritation
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use Ask a doctor or pharmacist before use
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- cough that occurs with too much phlegm mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
Ask a doctor or pharmacist before use if you are
taking any other oral nasal decongestant or stimulant.
When using this product do not use more than directed.
Stop use and ask a doctor if
- you get nervous, dizzy or sleepless
- symptoms do not get better within 7 days, or are accompanied by fever
- cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache.
These could be signs of a serious condition.
Directions
- Do not take more than 6 doses in any 24 hour period
- This adult strength product is not intended for use in children under 12 years of age
Age - adults and children 12 years and over Dose - 2 teaspoons every 4 hours
Age - children under 12 Dose - do not use
Other information
- each teaspoon contains: sodium 3 mg
- store at 20‐25 ° C (68‐77 ° F). Do not refrigerate.
- dosage cup provided
Inactive ingredients
anhydrous citric acid, FD and C red no. 40, glycerin, menthol, natural and artificial flavor, propylene glycol, purified water, sodium benzoate, sorbitol solution, sucralose
Product Label Tukol 504 DPL
Tukol®
DO NOT USE IF PRINTED SEAL UNDER CAP
IS TORN OR MISSING
Dextromethorphan HBr / Guaifenesin / Phenylephrine HCL
Expectorant/Cough Suppressant/Nasal Decongestant
Tukol®
Cough & Congestion
- Quiets Cough
- Thins and loosens phlegm
- Clears Nasal Congestion
Ages/ 12+ 4 FL OZ (118 mL)
2000005853
6 50066 00012 6
LOT No.
Exp.
Distributed by
Genomma Lab USA Inc.
Houston, TX 77027
BX-006 Rev. 03
Genoma Lab.®
rege