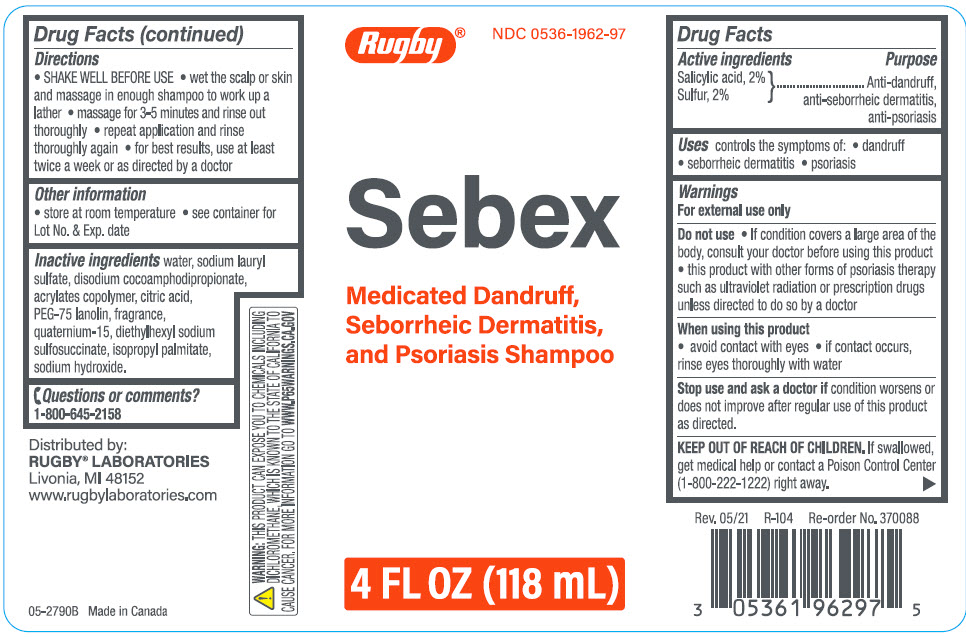
SEBEX ANTIDANDRUFF, ANTI-SEBORRHEIC DERMATITIS, ANTI-PSORIASIS- salicylic acid, sulfur shampoo
Rugby Laboratories
----------
| Active ingredients | Purpose |
Salicylic Acid, 2% }
Sulfur, 2% | Anti-dandruff, anti-seborrheic dermatitis, anti-psoriasis |
Uses
controls the symptoms of:
- dandruff
- seborrheic dermatitis
- psoriasis
Warnings
For external use only
Do not use
- If condition covers a large area of the body, consult your doctor before using this product
- this product with other forms of psoriasis therapy such as ultraviolet radiation or prescription drugs unless directed to do so by a doctor
When using this product
- avoid contact with eyes
- If contact occurs, rinse eyes thoroughly with water
Stop use and ask a doctor if condition worsens or does not improve after regular use of this product as directed.
KEEP OUT OF REACH OF CHILDREN. If swallowed, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
- SHAKE WELL BEFORE USE
- wet the scalp or skin and massage in enough shampoo to work up a lather
- massage for 3-5 minutes and rinse out thoroughly
- repeat application and rinse thoroughly again
- for best results, use at least twice a week or as directed by a doctor
Other information
- store at room temperature
- see container for Lot No. & Exp. date
Inactive ingredients
water, sodium lauryl sulfate, disodium cocoamphodipropionate, acrylates copolymer, citric acid, PEG-75 lanolin, fragrance, quaternium-15, diethylhexyl sodium sulfosuccinate, isopropyl palmitate, sodium hydroxide.
Questions or comments?
1-800-645-2158
Distributed by:
RUGBY® LABORATORIES
Livonia, MI 48152
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
Rugby®
NDC 0536-1962-97
Sebex
Medicated Dandruff,
Seborrheic Dermatitis,
and Psoriasis Shampoo
4 FL OZ (118 mL)