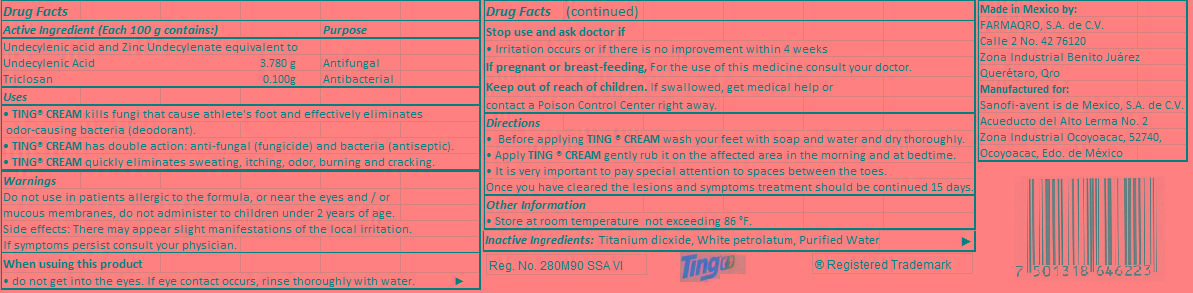
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
• TING® CREAM kills fungi that cause athlete's foot and effectively eliminates odor-causing bacteria (deodorant).
• TING® CREAM has double action: anti-fungal (fungicide) and bacteria (antiseptic).
• TING® CREAM quickly eliminates sweating, itching, odor, burning and cracking.
Warnings
Do not use in patients allergic to the formula, or near the eyes and/or mucous membranes, do not administer to children under 2 years of age.
Side effects: There may appear slight manifestations of the local irritation.
If symptoms persist consult your physician.
Directions
• Before applying TING ® CREAM wash your feet with soap and water and dry thoroughly.
• Apply TING ® CREAM gently rub it on the affected area in the morning and at bedtime.
• It is very important to pay special attention to spaces between the toes.
Once you have cleared the lesions and symptoms treatment should be continued 15 days.