OTC - ACTIVE INGREDIENT
Active Ingredient
(in each 5 mL = 1 teaspoon)
Ibuprofen 100 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
WARNINGS
Warnings
Allergy alert:
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin.
Symptoms may include:
-
■ hives ■ facial swelling
-
■ asthma (wheezing) ■ shock
-
■ skin reddening ■ rash ■ blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning:
This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chances are higher if your child:
-
■ has had stomach ulcers or bleeding problems
-
■ takes a blood thinning (anticoagulant) or steroid drug
-
■ takes other drugs containing an NSAID (aspirin, ibuprofen, naproxen, or others)
-
■ takes more or for a longer time than directed
Sore throat warning:
Severe or persistent sore throat or sore throat accompanied by high fever, headache, nausea, and vomiting may be serious. Consult doctor promptly. Do not use more than 2 days or administer to children under 3 years of age unless directed by doctor.
OTC - ASK DOCTOR
Ask a doctor before use if:
-
■ child has problems or serious side effects from taking pain relievers or fever reducers
-
■ stomach bleeding warning applies to your child
-
■ child has a history of stomach problems, such as heartburn
-
■ child has not been drinking fluids
-
■ child has lost a lot of fluid due to vomiting or diarrhea
-
■ child has high blood pressure, heart disease, liver cirrhosis, or kidney disease
-
■ child has asthma
-
■ child is taking a diuretic
OTC - STOP USE
Stop use and ask a doctor if:
-
■ child experiences any of the following signs of stomach bleeding
-
■ feels faint ■ vomits blood ■ has bloody or black stools
-
■ has stomach pain that does not get better
-
■ the child does not get any relief within the first day (24 hours) of treatment
-
■ fever or pain gets worse or lasts more than 3 days
-
■ redness or swelling is present in the painful area
-
■ any new symptoms appear
OTC - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
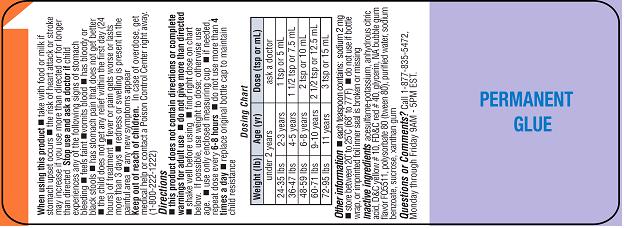
DOSAGE AND ADMINISTRATION
Directions
-
■ this product does not contain directions or complete warnings for adult use
-
■ do not give more than directed
-
■ shake well before using
-
■ find right dose on chart below (if possible, use weight to dose; otherwise use age)
-
■ use only enclosed measuring cup
-
■ if needed, repeat dose every 6-8 hours
-
■ do not use more than 4 times a day
-
■ replace original bottle cap to maintain child resistance
INACTIVE INGREDIENTS
acesulfame-potassium, anhydrous citric acid, D&C yellow # 10, FD&C red # 40, glycerin, NA bubble gum flavor FQ5511, polysorbate 80 (tween 80), purified water, sodium benzoate, sucrose, xanthan gum