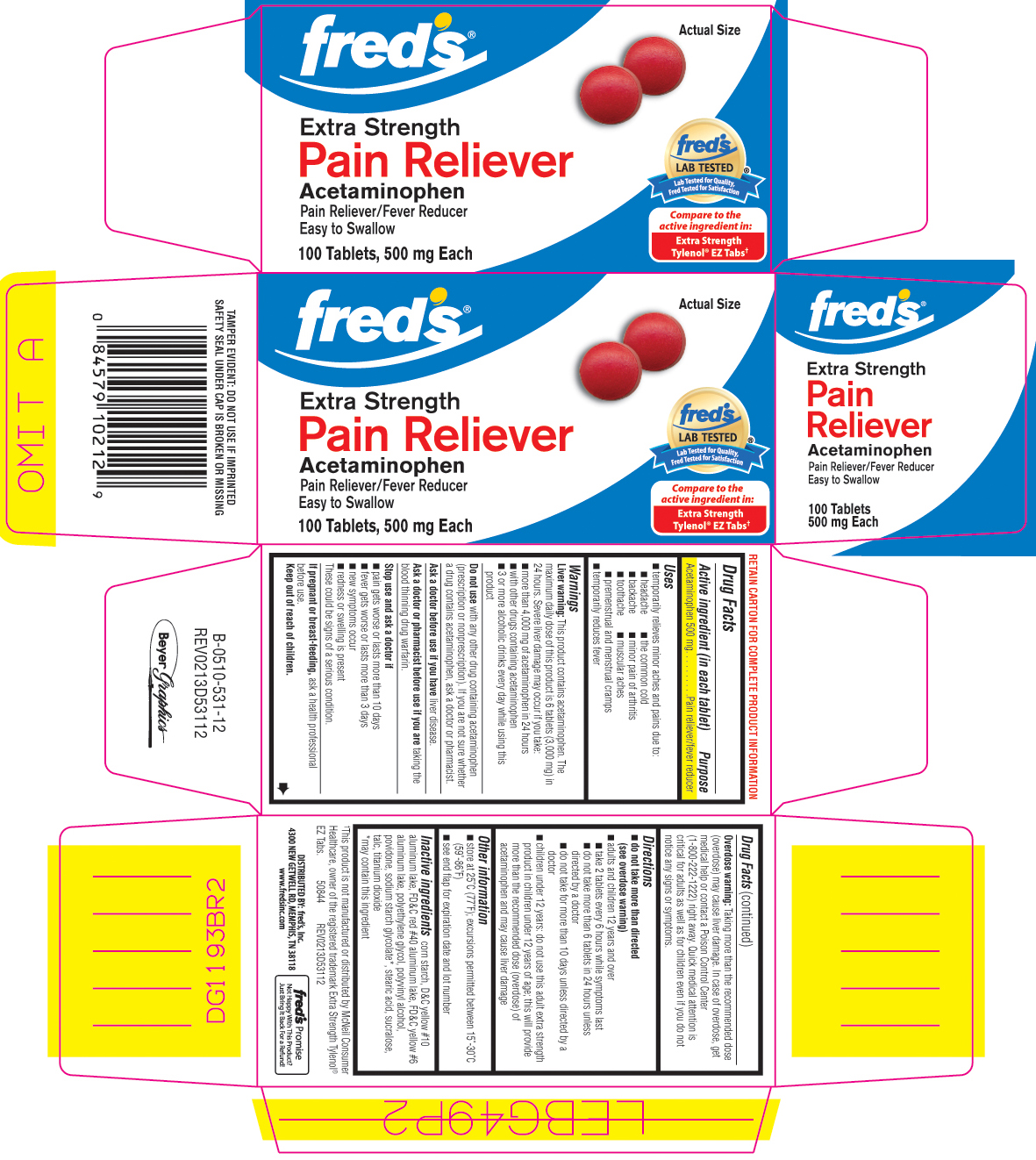
Uses
- temporarily relieves minor aches and pains due to:
- headache
- the common cold
- backache
- minor pain of arthritis
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. The maximum daily dose of this product is 6 tablets (3,000 mg) in 24 hours. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts ore than 3 days
- new symptoms occur
- redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center (1-8000-222-1222) right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
-
do not take more than directed
(see overdose warning) - adults and children 12 years and over
- take 2 tablets every 4 to 6 hours while symptoms last
- do not take more than 8 tablets in 24 hours
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: do not use this product in children under 12 years of age; this will provide more than the recommended dose (overdose) of acetaminophen and may cause liver damage
Other information
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- see end flap for expiration date and lot number
Inactive ingredients
D&C yellow #10 aluminum lake, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, polyethylene glycol, polyvinyl alcohol, povidone, sodium starch glycolate*, starch, stearic acid, sucralose, talc, titanium dioxide
*may contain this ingredient
Principal display panel
FRED'S®
Extra Strength Non-Aspirin
Pain Reliever
Pain Reliever/Fever Reducer
Acetaminophen
EZ Tabs
100 Tablets - 500 mg each
Tested Against The Active Ingredient In:
Extra Strength TYLENOL® EZ TABS†
†This product is not manufactured or distributed McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol® EZ Tabs.
50844 REV0213D53112
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Fred's 44-531