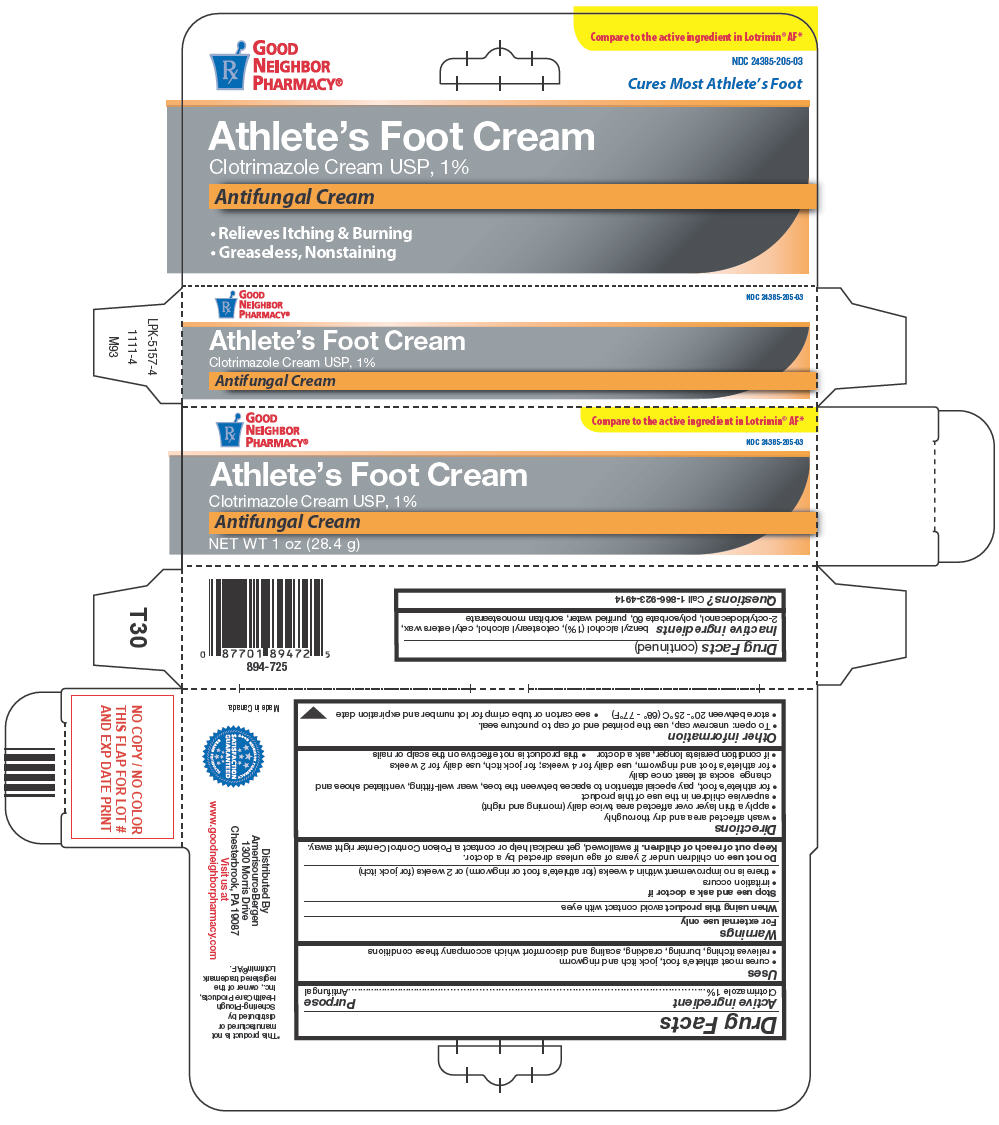
GOOD NEIGHBOR PHARMACY CLOTRIMAZOLE- clotrimazole cream
AmerisourceBergen
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Clotrimazole 1%
Uses
- cures most athlete's foot, jock itch and ringworm
- relieves itching, burning, cracking, scaling and discomfort which accompany these conditions
Warnings
For external use only
When using this product avoid contact with eyes
Stop use and ask a doctor if
- irritation occurs
- there is no improvement within 4 weeks (for athlete's foot or ringworm) or 2 weeks (for jock itch)
Do not use on children under 2 years of age unless directed by a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wash affected area and dry thoroughly
- apply a thin layer over affected area twice daily (morning and night)
- supervise children in the use of this product
- for athlete's foot, pay special attention to spaces between the toes, wear well-fitting, ventilated shoes and change socks at least once daily
- for athlete's foot and ringworm, use daily for 4 weeks; for jock itch, use daily for 2 weeks
- if condition persists longer, ask a doctor
- this product is not effective on the scalp or nails
Other information
- To open: unscrew cap, use the pointed end of cap to puncture seal.
- store between 20°- 25°C (68° - 77°F)
- see carton or tube crimp for lot number and expiration date
Inactive ingredients
benzyl alcohol (1%), cetostearyl alcohol, cetyl esters wax, 2-octyldodecanol, polysorbate 60, purified water, sorbitan monostearate
Questions?
Call 1-866-923-4914
Distributed By
AmerisourceBergen
1300 Morris Drive
Chesterbrook, PA 19087
PRINCIPAL DISPLAY PANEL - 28.4 g Tube Carton
Rx
GOOD
NEIGHBOR
PHARMACY®
Compare to the active ingredient in Lotrimin® AF*
NDC 24385-205-03
Athlete's Foot Cream
Clotrimazole Cream USP, 1%
Antifungal Cream
NET WT 1 oz (28.4 g)