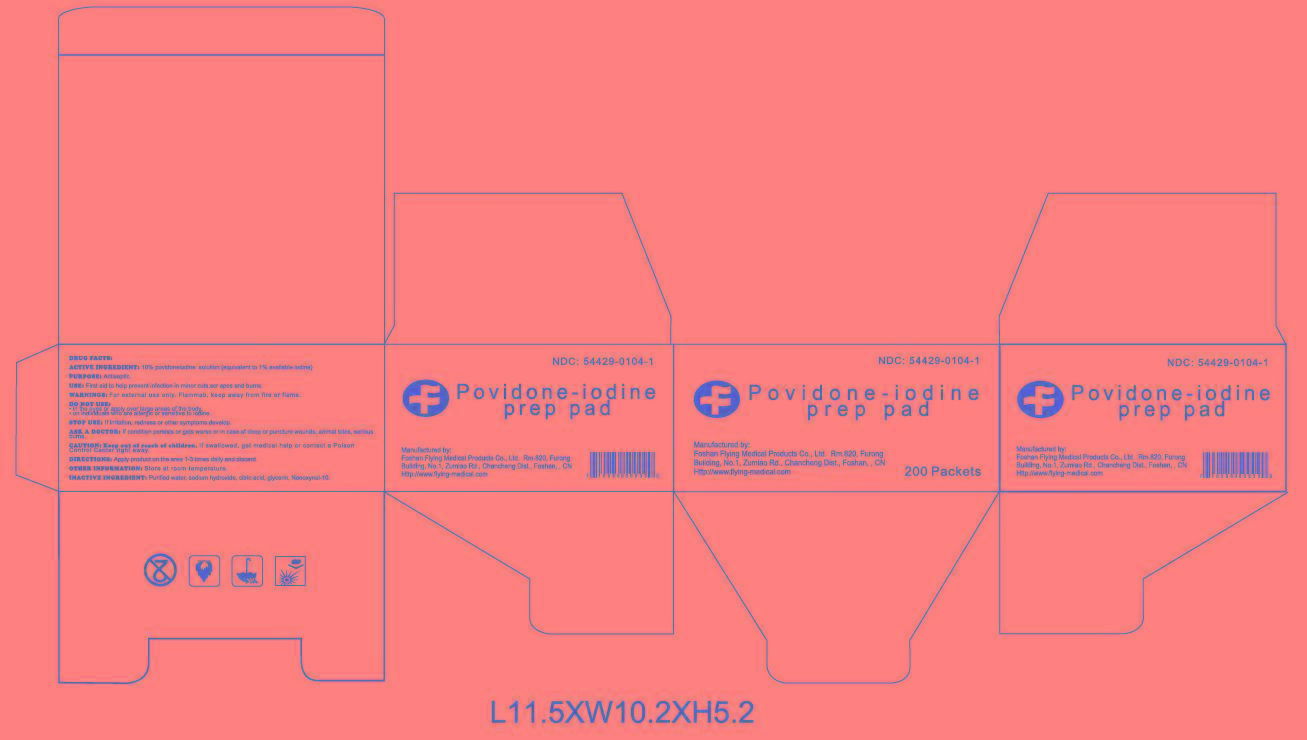
CAUTION: Keep out of reach of children
If swallowed, get medical help or contact Poison Control Center right away.
WARNINGS
For external use only. Flammable, keep away from fire or flame.
Do notuse
﹒in the eyes or apply over large areas of the body.
﹒on individuals who are allergic or sensitive to iodine.
Stop use
If irritation, redness or other symptoms develop.
Ask a doctor
If condition persists or gets worse or in case of deep or puncture wounds, animal bites, serious burns.
DRUG FACTS
Active ingredient
Povidone Iodine USP, 10% w/v
(equivalent to 1% titratable iodine)
Purpose
Antiseptic
Use
First aid to help prevent infection in minor cuts, scrapes, and burns
Warnings
For external use only. Flammable, keep away from fire or flame.
Do notuse
﹒in the eyes or apply over large areas of the body.
﹒on individuals who are allergic or sensitive to iodine.
Stop use
If irritation, redness or other symptoms develop.
Ask a doctor
If condition persists or gets worse or in case of deep or puncture wounds, animal bites, serious burns.
CAUTION: Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
Apply product on the area 1-3 times daily and discard.
Other information: Store at room temperature.
Inactive ingredients
ctric acid, glycerin, Nonoxynol-10, purified water, sodium hydroxide