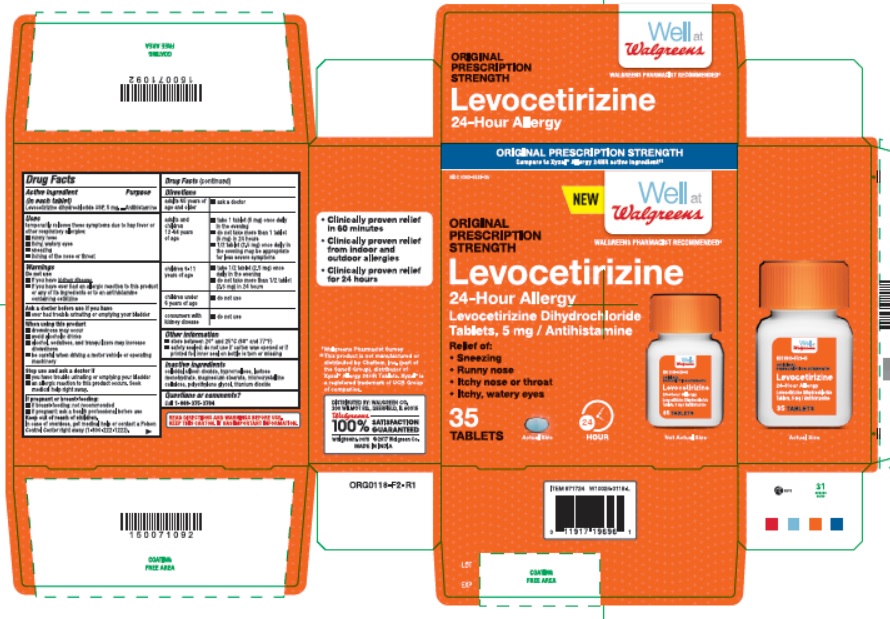
Uses
temporarily relieves these symptoms due to hay fever or other respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
- if you have kidney disease
- if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing cetirizine
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask doctor if
- you have trouble urinating or emptying your bladder
- an allergic reaction to this product occurs. Seek medical help right away.
Directions
| adults 65 years of age and older |
|
| adults and children 12-64 years of age |
|
| children 6-11 years of age |
|
| children under 6 years of age |
|
| consumers with kidney disease |
|
Other information
- store between 20° and 25°C (68° and 77°F)
- (bottles only) safety sealed: do not use if carton was opened or if printed foil inner seal on bottle is torn or missing
- (blister only) safety sealed: do not use if seal is broken or if individual blister unit is open or torn
Inactive ingredients
colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, titanium dioxide
Carton Label
ORIGINAL PRESCRIPTION STRENGTH
Compare to Xyzal®Allergy 24HR active ingredient‡‡
0363-5528-35
NEW Well at Walgreens
WALGREENS PHARMACIST RECOMMENDED‡
ORIGINAL
PRESCRIPTION
STRENGTH
Levocetirizine
24-Hour Allergy
Levocetirizine Dihydrochloride
Tablets, 5 mg / Antihistamine
Relief of:
- Sneezing
- Runny nose
- Itchy nose or throat
- Itchy, watery eyes
24 HOUR
35 TABLETS