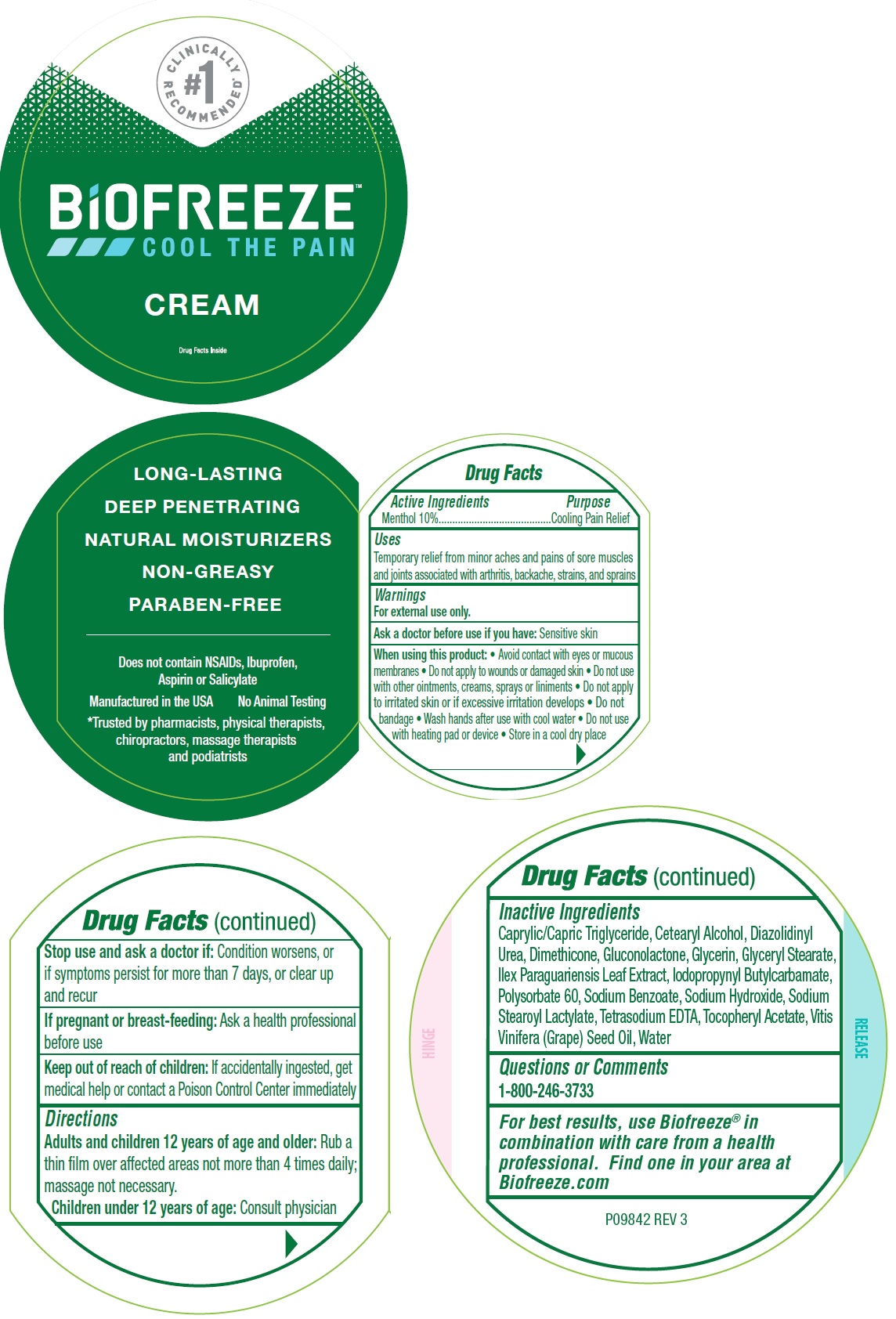
Uses
Temporary relief from minor aches and pains of sore muscles and joints associated with arthritis, backache, strains, and sprains
Warnings
For external use only.
When using this product:
- Avoid contact with eyes or mucous membranes
- Do not apply to wounds or damaged skin
- Do not use with other ointments, creams, sprays or liniments
- Do not apply to irritated skin or if excessive irritation develops
- Do not bandage
- Wash hands after use with cool water
- Do not use with heating pad or device
- Store in a cool dry place
Directions
- Adults and children 12 years of age and older: Rub a thin film over affected areas not more than 4 times daily; massage not necessary.
- Childern under 12 years of age: Consult physician
Inactive Ingredients
Caprylic/Capric Triglyceride, Cetearyl Alcohol, Diazolidinyl Urea, Dimethicone, Gluconolactone, Glycerin, Glyceryl Stearate, Ilex Paraguariensis Leaf Extract, Iodopropynyl butylcarbamate, Polysorbate 60, Sodium Benzoate, Sodium Hydroxide, Sodium Stearoyl Lactylate, Tetrasodium EDTA, Tocopheryl Acetate, Vitis Vinifera (Grape) seed Oil, Water