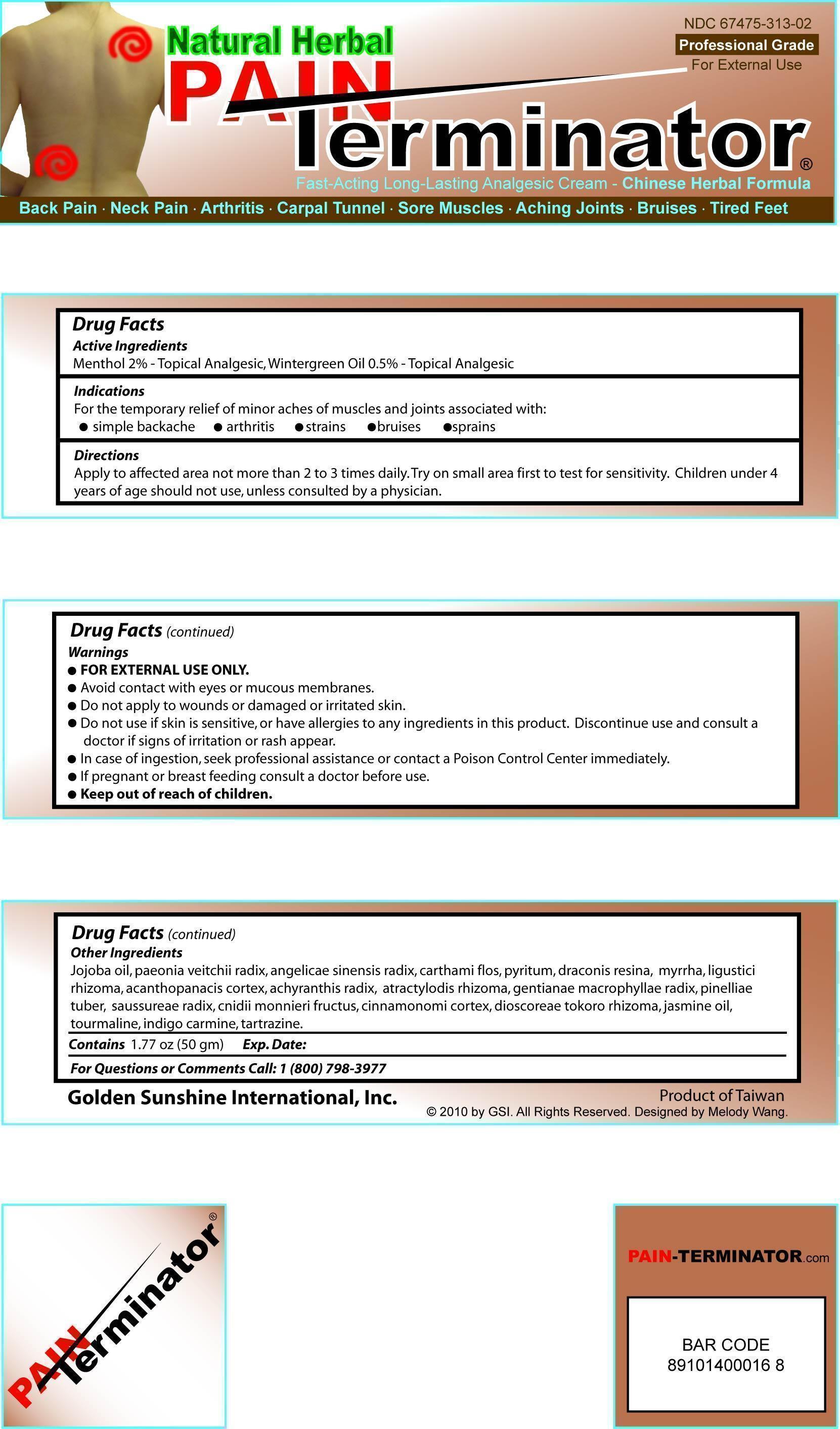
PAIN TERMINATOR ANALGESIC- topical analgesic cream
Golden Sunshine International, Inc.
----------
PAIN Terminator Analgesic Cream
Indications
For the temporary relief of minor aches of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Directions
Apply to affected area not more than 2 to 3 times daily. Try on small area first to test for
sensitivity. Children under 4 years of age should not use, unless consulted by a physician.
Warnings
- FOR EXTERNAL USE ONLY
- Avoid contac with eyes or mucous membranes.
- Do not apply to wounds or damaged or irritated skin.
- Do not use if skin is sensitive, or have allergies to any ingredients in this product. Discontinue use and consult a doctor if signs of irritation or rash appear.
- In case of ingestion, seek professional assistance or contact a Poison Control Center immediately.
Other Ingredients
Jojoba oil, paeonia veitchii radix, angelicae sinensis radix, carthami flos, pyritum, draconis resina, myrrha, ligustici rhizoma, acanthopanacis cortex, achyranthis radix, atractylodis rhizoma, gentianae macrophyllae radix, pinelliae tuber, saussureae radix, cnidii monnieri fructus, cinnamonomi cortex, dioscoreae tokoro rhizoma, jasmine oil, tourmaline
| PAIN TERMINATOR ANALGESIC
topical analgesic cream |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Golden Sunshine International, Inc. (098930857) |
| Registrant - Golden Sunshine International, Inc. <paragraph/></text> (098930857) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Golden Sunshine International, Inc. <paragraph/></text> | 098930857 | label(67475-313) | |
Revised: 11/2023
Document Id: fe37f549-0ad7-4a9a-8683-62cab7feaaae
Set id: ac38f9bd-6360-494c-b50d-5c5632743b91
Version: 4
Effective Time: 20231114
Golden Sunshine International, Inc.