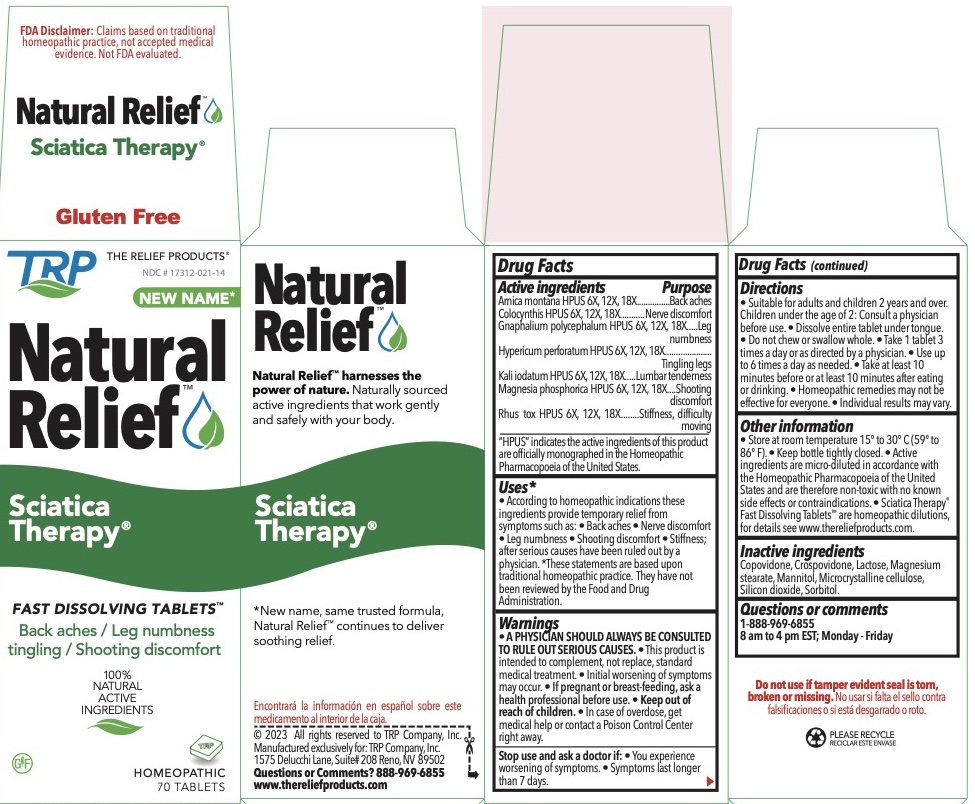
| Active Ingredients | Purpose | |
| Arnica Montana HPUS | 6x, 12x, 18x |
Back aches |
| Colocynthis HPUS | 6x, 12x, 18x |
Nerve discomfort |
| Gnaphalium polycephalum HPUS | 6X, 12x, 18x | Leg numbness |
| Hypericum perforatum HPUS | 6X, 12x, 18x | Tingling legs |
| Kali iodatum HPUS | 6X, 12x, 18x |
Lumbar tenderness |
| Magnesia phosphorica HPUS | 6X, 12x, 18x |
Shooting discomfort |
| Rhus toxicodendron HPUS | 6X, 12x, 18x |
Stiffness, Difficulty moving |
The letters HPUS indicate that the components of this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
Uses:
According to homeopathic indications these ingredients provide temporary relief from symptoms of Sciatica such as: • back aches • nerve discomfort • leg numbness • tingling legs • shooting discomfort • stiffness • tearing pain after serious causes have been ruled out by a physician.
* These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
Warnings:
A PHYSICIAN SHOULD ALWAYS BE CONSULTED TO RULE OUT SERIOUS CAUSES.
- This product is intended to complement, not replace, standard medical treatment.
- Initial worsening of symptoms may occur.
- A physician should always be consulted regarding Sciatica to rule out serious causes.
- In case of overdose, get medical help or contact a Poison Control Center right away.
Stop use and ask a doctor if: • Symptoms persist for more than 7 days or worsen, if new symptoms occur or if redness or swelling is present because these could be a sign of a serious illness.
Directions:
- Suitable for adults and children ages 2 and over.
- Children under the age of 2: consult a physician before use.
- Dissolve entire tablet under tongue.
- Do not chew or swallow whole.
- Take 1 tablet 3 times a day or as directed by a physician.
- Use up to 6 times a day as needed.
- Take at least 10 minutes before or at least 10 minutes after eating or drinking.
Other information:
- Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side effects.
- Store in a cool dark location.