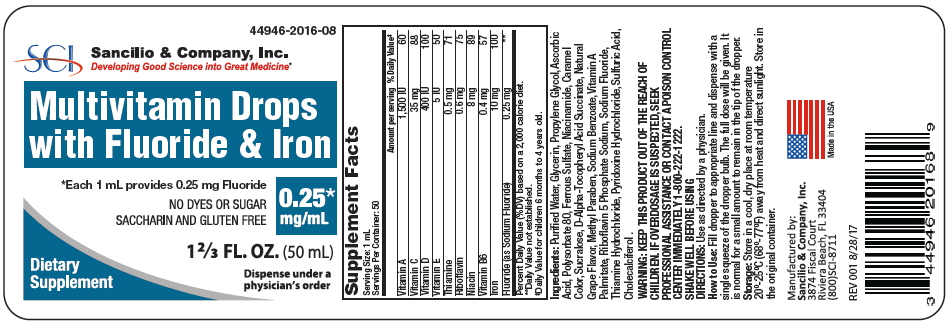
| Supplement Facts | ||
|---|---|---|
| Serving Size: 1 mL | ||
| Servings Per Container: 50 | ||
| Amount per serving | % Daily Value* | |
| Percent Daily Value (%DV) based on a 2,000 calorie diet. | ||
| Vitamin A | 1,500 IU | 60 |
| Vitamin C | 35 mg | 88 |
| Vitamin D | 400 IU | 100 |
| Vitamin E | 5 IU | 50 |
| Thiamine | 0.5 mg | 71 |
| Riboflavin | 0.6 mg | 75 |
| Niacin | 8 mg | 89 |
| Vitamin B6 | 0.4 mg | 57 |
| Iron | 10 mg | 100 |
| Fluoride (as Sodium Fluoride) | 0.25 mg | † |
Ingredients: Purified Water, Glycerin, Propylene Glycol, Ascorbic Acid, Polysorbate 80, Ferrous Sulfate, Niacinamide, Caramel Color, Sucralose, D-Alpha-Tocopheryl Acid Succinate, Natural Grape Flavor, Methyl Paraben, Sodium Benzoate, Vitamin A Palmitate, Riboflavin 5 Phosphate Sodium, Sodium Fluoride, Thiamine Hydrochloride, Pyridoxine Hydrochloride, Sulfuric Acid, Cholecalciferol .
WARNING
KEEP THIS PRODUCT OUT OF THE REACH OF CHILDREN. IF OVERDOSAGE IS SUSPECTED, SEEK PROFESSIONAL ASSISTANCE OR CONTACT A POISON CONTROL CENTER IMMEDIATELY 1-800-222-1222.
SHAKE WELL BEFORE USING
Storage
Store in a cool, dry place at room temperature 20°-25°C (68°-77°F) away from heat and direct sunlight. Store in the original container.
Manufactured by:
Sancilio & Company, Inc.
3874 Fiscal Court
Riviera Beach, FL 33404
(800)SCI-8711
Made in the USA
REV 001 8/28/17
PRINCIPAL DISPLAY PANEL - 0.25 mg/mL Bottle Label
44946-2016-08
SCI
Sancilio & Company, Inc.
Developing Good Science into Great Medicine®
Multivitamin Drops
with Fluoride & Iron
*Each 1 mL provides 0.25 mg Fluoride
NO DYES OR SUGAR
SACCHARIN AND GLUTEN FREE
0.25*
mg/mL
1 ⅔ FL. OZ. (50 mL)
Dietary
Supplement
Dispense under a
physician's order