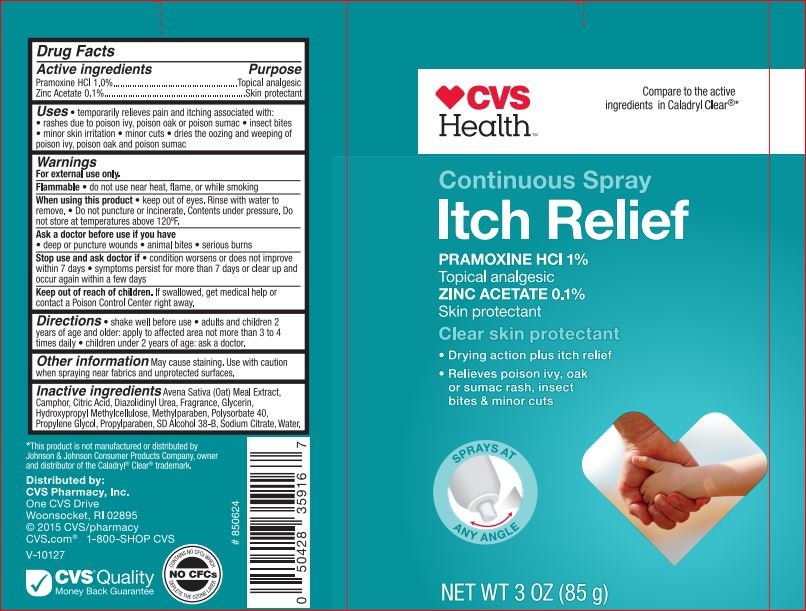
ITCH RELIEF CLEAR SKIN PROTECTANT CVS- pramoxine hcl, zinc acetate spray
CVS
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredient
Pramoxine HCl 1%
Zinc Acetate 0.1%
Uses
- Temporarily relieves pain and itching asociated with:
- rashes due to poison ivy, poison oak or poison sumac.
- insect bites.
- minor skin irritation.
- minor cuts.
- dries the oozing and weeping of poison ivy, poison oak and poison sumac.
Warnings
For external use only.
Flammable: Do not use near heat, flame, or while smoking.
When using this product
- keep out of eyes. Rinse with water to remove.
- Do not puncture or incinerate. Contents under pressure. Do no store at temperatures above 120F.
Stop use and ask a doctor if
- condition worsens or does not improve within 7 days.
- symptoms persist for more than 7 days or clear up and occur again with in a few days.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- shake well before use.
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily.
- children under 2 years of age: ask a doctor.
Inactive ingredients
SD Alcohol 38-B, Avena Sative (Oat Meal) Extract, Camphor, Citric Acid, Diazolidinyl Urea, Fragrance, Glycerin, Hypromellose, Methylparaben,Polysorbate 40, Propylene Glycol, Propylparaben, Sodium Citrate, Water.
CVS Continuous Spray Itch Relief
Clear Skin Protectant
1% Pramoxine HCl
0.1% Zinc Acetate