INTRALIPID - soybean oil emulsion
Baxter Healthcare CORP
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Intralipid 10% emulsion for infusion
Intralipid 20% emulsion for infusion
Qualitative and quantitative composition
Intralipid 10%
1000 ml of the emulsion contains: Purified soybean oil 100 g,
purified egg phospholipids, glycerol anhydrous, sodium hydroxide, water for injections pH appr 8
Osmolality: 300 mosm/kg water
Energy content: 4.6 MJ (1100 kcal) / 1000 ml
Organic phosphate content: 15 mmol / 1000 ml
Intralipid 20%
1000 ml of the emulsion contains: Purified soybean oil 200 g,
purified egg phospholipids, glycerol anhydrous, sodium hydroxide, water for injections pH appr 8
Osmolality: 350 mosm/kg water
Energy content: 8.4 MJ (2000 kcal) / 1000 ml
Organic phosphate content: 15 mmol / 1000 ml
PHARMACOLOGICAL PROPERTIES
Pharmacodynamic properties
Intralipid provides essential and non-essential long-chain fatty acids for energy metabolism and apposition in cell membranes.
Intralipid in the recommended dosage does not cause any haemodynamic changes. No clinically significant changes in pulmonary function have been described when Intralipid is used properly. The transient increase in liver enzymes seen in some patients on TPN including Intralipid is reversible and disappears when TPN is interrupted. Similar changes are seen also in parenteral nutrition without fat emulsions.
Pharmacokinetic properties
Intralipid has biological properties similar to those of endogenous chylomicrons. Unlike chylomicrons, Intralipid does not contain cholesterol esters or apolipoproteins, while its phospholipid content is significantly higher.
Intralipid is eliminated from the circulation via the same pathway as endogenous chylomicrons, at least early on in the catabolism. The exogenous fat particle is hydrolysed in the circulation and taken up by LDL receptors peripherally and by the liver. The elimination rate is determined by the composition of the fat particles, the nutritional status, the disease and the rate of infusion. In healthy volunteers the maximum clearance rate of Intralipid after fasting overnight is equivalent to 3.8+1.5 g of triglycerides/kg body weight/24 hours.
Both the elimination and the oxidation rates are dependent on the patient’s clinical condition; elimination is faster and utilisation is increased in postoperative patients and in trauma, while patients with renal failure and hypertriglyceridaemia show lower utilisation of exogenous fat emulsions.
CLINICAL PARTICULARS
Therapeutic indications
Intralipid is indicated in patients needing intravenous nutrition to supply energy and essential fatty acids. Intralipid is also indicated in patients with essential fatty acid deficiency (EFAD) who cannot maintain or restore a normal essential fatty acid pattern by oral intake.
Posology and method of administration
The ability to eliminate Intralipid should govern the dosage and infusion rate. See below Fat elimination.
Dosage Intralipid 10%
1g triglycerides corresponds to 10 ml Intralipid 10%.
Adults. The recommended maximum dosage is 3 g triglycerides/kg body weight/day. Within this upper limit, Intralipid can be given to contribute up to 70% of the energy requirements, also in patients with highly increased energy requirements. The infusion rate for Intralipid 10% should not exceed 500 ml in 5 hours.
Neonates and infants. The recommended dosage range in neonates and infants is 0.5-4 g triglycerides/ kg bw/day. The rate of infusion should not exceed 0.17 g triglycerides/kg bw/hour (4 g in 24 hours). In prematures and low birthweight neonates, Intralipid should preferably be infused continuously over 24 hours. The initial dosage should be 0.5-1 g/kg bw/day followed by a successive increase by 0.5-1 g/kg bw/day up to 2g/kg bw/day. Only with close monitoring of serum triglyceride concentration, liver tests and oxygen saturation may the dosage be increased to 4 g/kg bw/day. The rates given are maximum rates and no attempt should be made to exceed these in order to compensate for missed doses.
Dosage Intralipid 20%
1g triglycerides corresponds to 5 ml Intralipid 20%.
Adults. The recommended maximum dosage is 3 g triglycerides/ kg body weight/day. Within this upper limit, Intralipid can be given to contribute up to 70% of the energy requirements, also in patients with highly increased energy requirements. The infusion rate for Intralipid 20% should not exceed 500 ml in 5 hours.
Neonates and infants. The recommended dosage range in neonates and infants is 0.5-4 g triglycerides/ kg bw/day. The rate of infusion should not exceed 0.17 g triglycerides/kg bw/hour (4 g in 24 hours). In prematures and low birthweight neonates, Intralipid should preferably be infused continuously over 24 hours. The initial dosage should be 0.5-1 g/kg bw/day followed by a successive increase by 0.5-1 g/kg bw/day up to 2g/kg bw/day. Only with close monitoring of serum triglyceride concentration, liver tests and oxygen saturation may the dosage be increased to 4 g/kg bw/day. The rates given are maximum rates and no attempt should be made to exceed these in order to compensate for missed doses.
Essential fatty acid deficiency (EFAD). To prevent or correct essential fatty acid deficiency, 4 to 8% of the nonprotein energy should be supplied as Intralipid to provide sufficient amounts of linoleic and linolenic acid. When EFAD is associated with stress, the amount of Intralipid needed to correct the deficiency may be substantially increased.
FAT ELIMINATION
Adults. The ability to eliminate fat should be closely monitored in patients with conditions mentioned in section “Special warnings and special precautions for use”, and in patients given Intralipid for more than one week. This is done by collecting a blood sample after a fat-free clearance period of 5-6 hours. Blood cells are then separated from plasma by centrifugation. If the plasma is opalescent, the infusion should be postponed. The sensitivity of this method is such that hypertriglyceridaemia can pass undetected.
Therefore, it is recommended that serum triglyceride concentrations should be measured in patients who are likely to have impaired fat tolerance.
Neonates and infants. The ability to eliminate fat should be tested regularly in neonates and infants. Measuring serum triglyceride levels is the only reliable method.
Contra-indications
Intralipid is contraindicated in patients with acute shock and in patients with severe hyperlipemia. Severe liver insufficiency. Hemophagocytotic syndrome. Hypersensitivity to egg-, soya- or peanut protein or to any of the active substances or excipients.
Special warnings and special precautions for use
Intralipid should be given with caution in conditions of impaired lipid metabolism as in renal insufficiency, uncompensated diabetes mellitus, pancreatitis, impaired liver function, hypothyroidism (if hypertri-glyceridemic) and sepsis. If Intralipid is given to patients with these conditions, close monitoring of the serum triglyceride concentration is obligatory.
This medicinal product contains soya-bean oil and egg phospholipids, which may rarely cause allergic reactions. Cross allergic reactions have been observed between soybean and peanut.
Intralipid should be given with caution to neonates and prematures with hyperbilirubinemia and cases with suspected pulmonary hypertension. In neonates, particularly prematures on long term parenteral nutrition, platelet count, liver tests and serum triglyceride concentration should be monitored.
Intralipid may interfere with certain laboratory measurements (bilirubin, lactate dehydrogenase, oxygen saturation, Hb etc) if blood is sampled before fat has been adequately cleared from the blood stream. Fat is cleared after a fat free interval of 5-6 hours in most patients.
Interaction with other medicaments and other forms of interaction
Some drugs, like insulin, may interfere with the body’s lipase system. This kind of interaction seems, however, to be of only limited clinical importance.
Heparin in clinical doses causes a transient increase in lipolysis in plasma, resulting in a transient decrease in triglyceride clearance due to depletion of lipoprotein lipase.
Soybean oil has a natural content of vitamin K1. This is considered important only for patients treated with coumarin derivatives, which interfere with vitamin K1.
Pregnancy and lactation
No adverse reactions connected with pregnancy and lactation have been reported.
Effects on ability to drive and use machines
No effects on the ability to drive and operate machines are to be expected.
Undesirable effects
Intralipid infusion may cause a rise in body temperature and, less frequently, shivering, chills and nausea/vomiting (incidence<1%).
Reports of other adverse events in conjunction with Intralipid infusion are extremely rare, less than one adverse event per one million infusions.
System Organ Class Frequency Symptom
according to WHO
Body as a whole Uncommon Headache
general disorders (>1/1000, <1/100) Rise in body
temperature,
shivering, chills,
tiredness
Very rare Anaphylactic
(<1/10 000) reaction
Cardiovascular disorders Very rare Circulatory effects
(<1/10 000) (e.g. hyper/hypotension)
Gastrointestinal disorders Uncommon Abnormal pain
(>1/1000, <1/100) Nausea, vomiting
Liver & biliary system Very rare Transient increase
disorders (<1/10 000) in liver function
test
Musculoskeletal, Very rare Abdominal pain
connective tissue and (<1/10 000)
bone disorders
Platelet, bleeding & Very rare Thrombocytopenia
clotting disorders (<1/10 000)
Red blood cell disorders Very rare Haemolysis,
(<1/10 000) reticulocytosis
Reproductive disorders, Very rare Priapism
male (<1/10 000)
Skin and appendages Very rare Rash, urticaria
disorders (<1/10 000)
Trombocytopenia has been reported in association with prolonged treatment with Intralipid in infants.
Transient increase in liver function tests after prolonged intravenous nutrition with or without Intralipid have also been noted.
Increased cholesterol has been observed with infants after long term treatment with Intralipid 10%. The reasons are not clear
at present.
Fat overload syndrome. An impaired capacity to eliminate Intralipid may lead to the fat overload syndrome as a result of overdosage. However, this syndrome may appear also at recommended rates of infusion in association with a sudden change in the patient’s clinical condition, such as renal function impairment or infection. The fat overload syndrome is characterised by hyperlipemia, fever, fat infiltration and disorders in various organs and coma. All symptoms are usually reversible if the infusion of Intralipid is discontinued.
Overdose
See Undesirable effects, “Fat overload syndrome”. Severe overdose of fat emulsions containing triglycerides can, especially if carbohydrates are not administered simultaneously, lead to acidosis.
Instructions for use - Fresenius Kabi infusion bag
1.
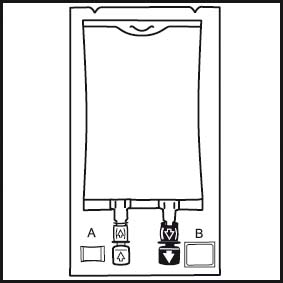
1. The integrity indicator (Oxalert™) A should be inspected before removing the overwrap. If the indicator is black the overwrap is damaged and the product should be discarded.
2.
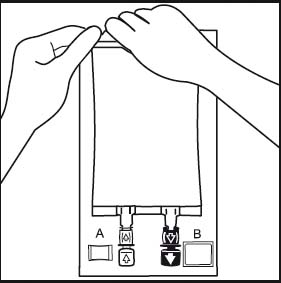
2. Remove the overwrap by tearing at the notch and pulling down along the container. The Oxalert™ sachet A and the oxygen absorber B should be disposed.
3.
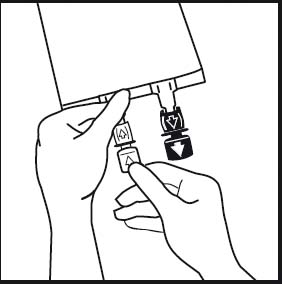
3. If additives are to be used break off the tamper-evident arrow flag from the white additive port. If no additives are to be used go to figure 5.
4.
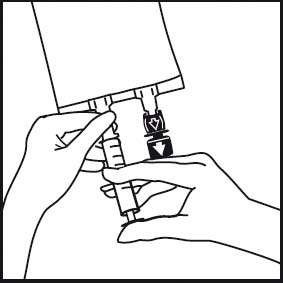
4. Insert the needle horizontally through the centre of the septum of the additive port and inject the additives (with known compatibility). Use syringes with needles of 18 - 23 gauge and a length of max. 40 mm.
5.
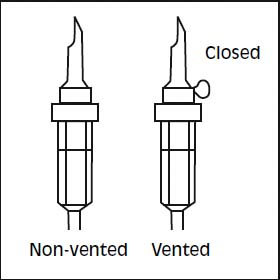
5. Use a non-vented infusion set or close the air vent on a vented set. Follow the instructions for use for the infusion set. Use a spike with diameter as specified in ISO 8536-4, 5.6 +/- 0.1 mm.
6.
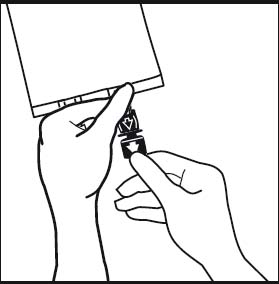
6. Break off the tamper-evident arrow flag from the blue infusion port.
7.
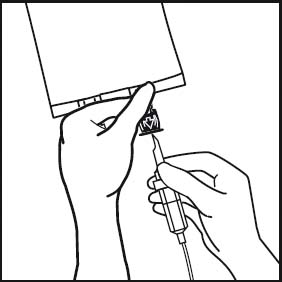
7. Hold the base of the infusion port. Insert the spike through the infusion port, by rotating your wrist slighly until the spike is inserted.
8.
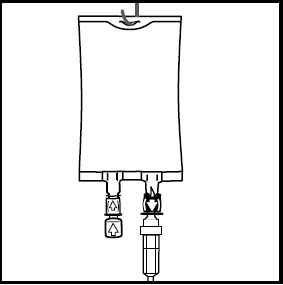
8. Hang the bag in the hanger cut and start infusion.
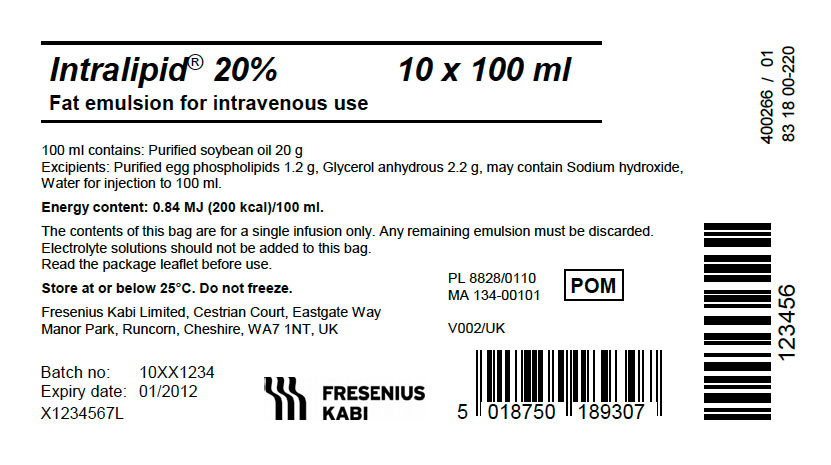
PACKAGE LABEL - PRINCIPAL DISPLAY - Intralipid 20% 100 mL Bag Label
Intralipid® 20% 100 mL
Fat emulsion for intravenous use

PACKAGE LABEL - PRINCIPAL DISPLAY - Intralipid 20% 100 mL Bag Carton Panel
Intralipid® 20% 10 x 100 mL
Fat emulsion for intravenous use
PHARMACEUTICAL PARTICULARS
Incompatibilities
Intralipid can only be mixed with other medicinal products for which compatibility has been documented.
Shelf life
Shelf life of the product as packaged for sale. 24 months
Shelf life after first opening the container. The emulsion should be used directly due to the risk of microbiological contamination. Any unused emulsion should be discarded.
Shelf life after addition or mixing according to directions.
When additions are made to infusion solution, the infusion should be completed within 24 hours.
Special precautions for storage
Store below 25°C. Do not freeze.
After addition of other nutritional elements
Mixing in plastic bag (phthalate free film): Mixtures aseptically prepared in a controlled and validated aseptic area should be used within 7 days after preparation. The mixtures can be stored up to 6 days in a refrigerator (2-8°C) followed by an infusion period of up to 24 hours.
Nature and contents of container
Infusion bottle Type II glass and butyl rubber stopper.
- All packaging components are latex- and PVC-free.
Pack sizes:
Intralipid 10% 100 ml, 500 ml
Intralipid 20% 100 ml, 250 ml, 500 ml, 1000 ml
Infusion bag
The container consists of an inner bag and an overpouch. An oxygen absorber and integrity indicator are placed between the inner bag and the overpouch. The inner bag is the primary container for Intralipid.
The overpouch provides protection during storage by contributing with barrier properties towards water and oxygen to the Intralipid container system.
The oxygen absorber will absorb and bind oxygen remaining between the inner bag and the overpouch. The integrity indicator will react with free oxygen and change from clear to black in case of a damaged overpouch.
- The inner bag is made of a multilayer polymer film, Biofine
- The Biofine inner bag film consists of poly(propylene/
ethylene) copolymer and thermoplastic elastomers (SEBS and
SIS). The infusion and additive ports are made of polypropylene
and a thermoplastic elastomer (SEBS) equipped with synthetic
polyisoprene stoppers.
- The oxygen barrier overpouch consists of polyolefin and
polyethylene terephtalate or polyolefin, polyethylene
terephtalate and poly(ethyl vinyl) alcohol (EVOH).
- The oxygen absorber consists of iron powder in a polymer
sachet.
- The integrity indicator (OxalertTM) consists of an oxygen
sensitive solution in a polymer sachet.
- All packaging components are latex- and PVC-free.
Pack sizes:
Intralipid 10% 100 ml, 500 ml
Intralipid 20% 100 ml, 250 ml, 500 ml
Instructions for use/handling
Do not use if the package is damaged.
Infusion bag: The integrity indicator (OxalertTM) should be inspected before removing the overpouch. If the indicator is black, oxygen has penetrated the overpouch and the product should be discarded.
The overpouch, the oxygen absorber and the integrity indicator should be discarded after opening of the overpouch.
Additions should be made aseptically. Single administration of electrolyte solutions to Intralipid should not be made. Only medicinal, nutritional or electrolyte solutions for which compatibility has been documented may be added as directed. Compatibility data are available from the manufacturer for a number of mixtures. The left over contents of opened bottles / bags should be discarded and not saved for later use.
This leaflet was last revised 08/2012
| INTRALIPID
soybean oil emulsion |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Baxter Healthcare CORP (005083209) |
| Registrant - Fresenius Kabi Gmbh Deutschland (506719546) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fresenius Kabi AB Uppsala | 559785113 | ANALYSIS(0338-0519) , MANUFACTURE(0338-0519) | |