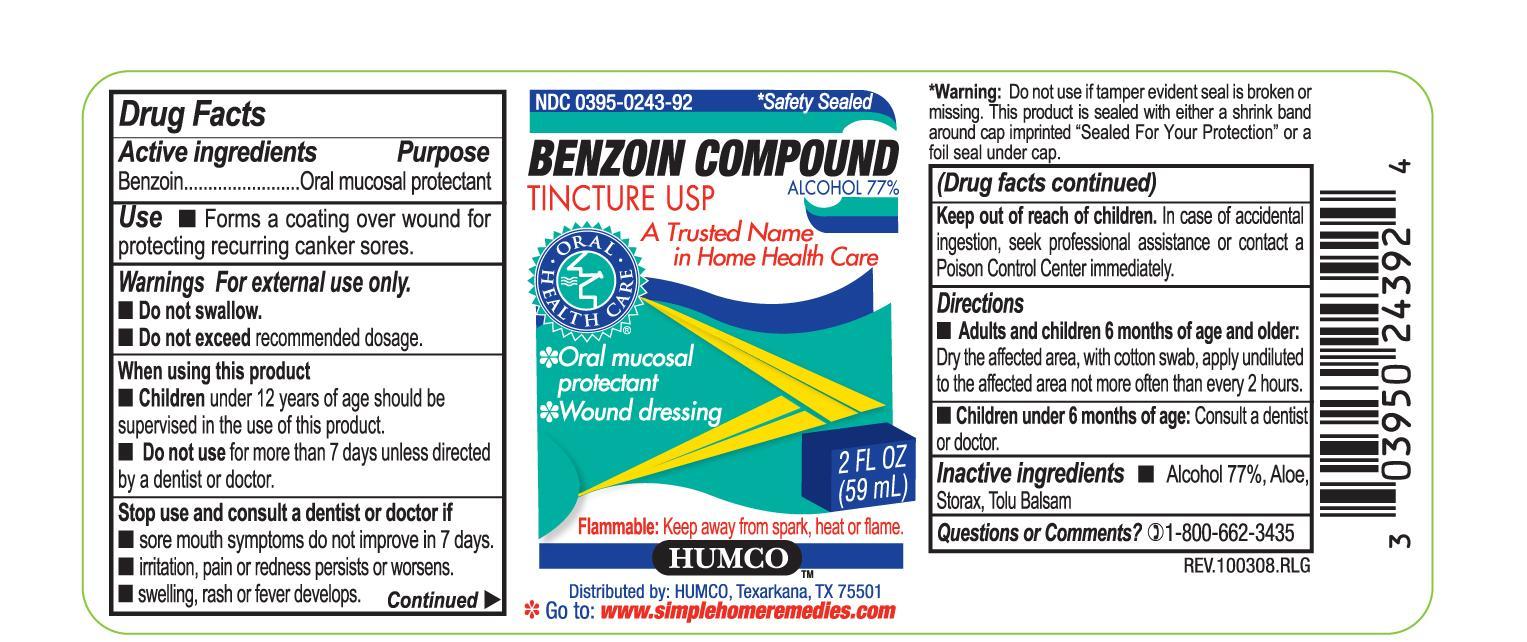
Active Ingredient
Benzoin
Purpose
Oral mucosal protectant
Use
Forms a coating over wound for protecting recurring canker sores
Warnings
For external use only. Do not swallow. Do not exceed recommended dosage.
When using this product
Children under 12 years of age should be supervised in the use of this product.
Do not use for more than 7 days unless directed by a dentist or doctor.
Stop use and consult a dentist or doctor if
sore mouth symptoms do not improve in 7 days. irritation, pain or redness persists or worsens. swelling, rash or fever develops.
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
Adults and children 6 months of age and older: Dry the affected area, with cotton swab, apply undiluted to the affected area not more often than every 2 hours.
Children under 6 months of age: Consult a dentist or doctor.
Other information
Flammable: Keep away from spark, heat or flame.
Inactive Ingredients
Alcohol 77%, Aloe, Storax, Tolu Balsam
Principal Display Panel - 16oz


Principal Display Panel - 2 oz


Humco Holding Group, Inc.




