DESCRIPTION:
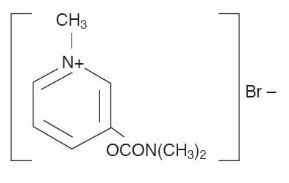
MESTINON (pyridostigmine bromide) is an orally active cholinesterase inhibitor. Chemically, pyridostigmine bromide is 3-hydroxy-1-methylpyridinium bromide dimethylcarbamate. Its structural formula is:
MESTINON is available in the following forms: Oral Solution containing 60 mg pyridostigmine bromide per teaspoonful in a vehicle containing 5% alcohol, glycerin, lactic acid, sodium benzoate, sorbitol, sucrose, FD&C Red No. 40, FD&C Blue No. 1, flavors and water. Tablets containing 60 mg pyridostigmine bromide; each tablet also contains lactose, silicon dioxide and stearic acid. TIMESPAN tablets containing 180 mg pyridostigmine bromide; each tablet also contains carnauba wax, corn-derived proteins, isopropyl alcohol, magnesium stearate, silica gel, tribasic calcium phosphate and water.
ACTIONS:
MESTINON inhibits the destruction of acetylcholine by cholinesterase and thereby permits freer transmission of nerve impulses across the neuromuscular junction. Pyridostigmine is an analog of neostigmine (Prostigmin), but differs from it in certain clinically significant respects; for example, pyridostigmine is characterized by a longer duration of action and fewer gastrointestinal side effects.
CONTRAINDICATIONS:
MESTINON is contraindicated in mechanical intestinal or urinary obstruction, and particular caution should be used in its administration to patients with bronchial asthma. Care should be observed in the use of atropine for counteracting side effects, as discussed below.
WARNINGS:
Although failure of patients to show clinical improvement may reflect underdosage, it can also be indicative of overdosage. As is true of all cholinergic drugs, overdosage of MESTINON may result in cholinergic crisis, a state characterized by increasing muscle weakness which, through involvement of the muscles of respiration, may lead to death. Myasthenic crisis due to an increase in the severity of the disease is also accompanied by extreme muscle weakness, and thus may be difficult to distinguish from cholinergic crisis on a symptomatic basis. Such differentiation is extremely important, since increases in doses of MESTINON or other drugs of this class in the presence of cholinergic crisis or of a refractory or “insensitive” state could have grave consequences. Osserman and Genkins1 indicate that the differential diagnosis of the two types of crisis may require the use of Tensilon (edrophonium chloride) as well as clinical judgment. The treatment of the two conditions obviously differs radically. Whereas the presence of myasthenic crisis suggests the need for more intensive anticholinesterase therapy, the diagnosis of cholinergic crisis, according to Osserman and Genkins1, calls for the prompt withdrawal of all drugs of this type. The immediate use of atropine in cholinergic crisis is also recommended.
Atropine may also be used to abolish or obtund gastrointestinal side effects or other muscarinic reactions; but such use, by masking signs of overdosage, can lead to inadvertent induction of cholinergic crisis.
For detailed information on the management of patients with myasthenia gravis, the physician is referred to one of the excellent reviews such as those by Osserman and Genkins2, Grob3 or Schwab.4,5
PRECAUTION:
Pyridostigmine is mainly excreted unchanged by the kidney.6,7,8 Therefore, lower doses may be required in patients with renal disease, and treatment should be based on titration of drug dosage to effect.6,7
ADVERSE REACTIONS:
The side effects of MESTINON are most commonly related to overdosage and generally are of two varieties, muscarinic and nicotinic. Among those in the former group are nausea, vomiting, diarrhea, abdominal cramps, increased peristalsis, increased salivation, increased bronchial secretions, miosis and diaphoresis. Nicotinic side effects are comprised chiefly of muscle cramps, fasciculation and weakness. Muscarinic side effects can usually be counteracted by atropine, but for reasons shown in the preceding section the expedient is not without danger. As with any compound containing the bromide radical, a skin rash may be seen in an occasional patient. Such reactions usually subside promptly upon discontinuance of the medication.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION:
MESTINON is available in three dosage forms:
Oral Solution -
raspberry-flavored, containing 60 mg pyridostigmine bromide per teaspoonful (5 mL). This form permits accurate dosage adjustment for children and “brittle” myasthenic patients who require fractions of 60 mg doses. It is more easily swallowed, especially in the morning, by patients with bulbar involvement.
TIMESPAN tablets -
each containing 180 mg pyridostigmine bromide. This form provides uniformly slow release, hence prolonged duration of drug action; it facilitates control of myasthenic symptoms with fewer individual doses daily. The immediate effect of a 180 mg TIMESPAN tablet is about equal to that of a 60 mg Conventional tablet; however, its duration of effectiveness, although varying in individual patients, averages 2½ times that of a 60 mg dose.
Dosage:
The size and frequency of the dosage must be adjusted to the needs of the individual patient.
Oral Solution and Conventional tablets -
The average dose is ten 60 mg tablets or ten 5 mL teaspoonfuls daily, spaced to provide maximum relief when maximum strength is needed. In severe cases as many as 25 tablets or teaspoonfuls a day may be required, while in mild cases one to six tablets or teaspoonfuls a day may suffice.
TIMESPAN tablets -
One to three 180 mg tablets, once or twice daily, will usually be sufficient to control symptoms; however, the needs of certain individuals may vary markedly from this average. The interval between doses should be at least 6 hours. For optimum control, it may be necessary to use the more rapidly acting regular tablets or oral solution in conjunction with TIMESPAN therapy.
NOTE: For information on a diagnostic test for myasthenia gravis, and for the evaluation and stabilization of therapy, please see product literature on Tensilon (edrophonium chloride).
HOW SUPPLIED:
Oral Solution, 60 mg pyridostigmine bromide per teaspoonful (5 mL) and 5% alcohol - bottles of 1 pint (473 mL) (NDC 0187-3012-20).
Tablets, are available as white, flat-faced tablets containing 60 mg pyridostigmine bromide in bottles of 100 (NDC 0187-3010-30). Each tablet is engraved “MESTINON 60 V” on one side and is quadrisect scored on the other.
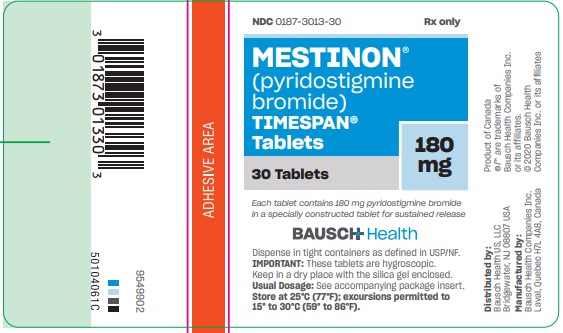
TIMESPAN tablets, are available as light straw-colored, capsule-shaped tablets containing 180 mg pyridostigmine bromide in bottles of 30 (NDC 0187-3013-30).
Each tablet is engraved “MES V 180” on one side and is single-scored on the other.
Note: Because of the hygroscopic nature of the TIMESPAN tablets, mottling may occur. This does not affect their efficacy.
REFERENCES:
- 1.
- Osserman KE, Genkins G. Studies in myasthenia gravis: Reduction in mortality rate after crisis. JAMA. Jan 1963; 183:97-101.
- 2.
- Osserman KE, Genkins G. Studies in myasthenia gravis. NY State J Med. June 1961; 61:2076-2085.
- 3.
- Grob D. Myasthenia gravis. A review of pathogenesis and treatment. Arch Intern Med. Oct 1961; 108:615-638.
- 4.
- Schwab RS. Management of myasthenia gravis. New Eng J Med. Mar 1963; 268:596-597.
- 5.
- Schwab RS. Management of myasthenia gravis. New Eng J Med. Mar 1963; 268:717-719.
- 6.
- Cronnelly R, Stanski DR, Miller RD, Sheiner LB. Pyridostigmine kinetics with and without renal function. Clin Pharmacol Ther. 1980; 28: No. 1, 78-81.
- 7.
- Miller RD. Pharmacodynamics and pharmacokinetics of anticholinesterase. In: Ruegheimer E, Zindler M, ed. Anesthesiology. (Hamburg, Germany: Congress; Sep 14-21, 1980; 222-223.) (Int Congr. No. 538), Amsterdam, Netherlands: Excerpta Medica; 1981.
- 8.
- Breyer-Pfaff U, Maier U, Brinkmann AM, Schumm F. Pyridostigmine kinetics in healthy subjects and patients with myasthenia gravis. Clin Pharmacol Ther. 1985; 5:495-501.
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
-
Manufactured by:
Bausch Health Companies Inc.
Laval, Quebec H7L 4A8, Canada
®/™ are trademarks of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
9600203 50102987F
Rev. 12/2020
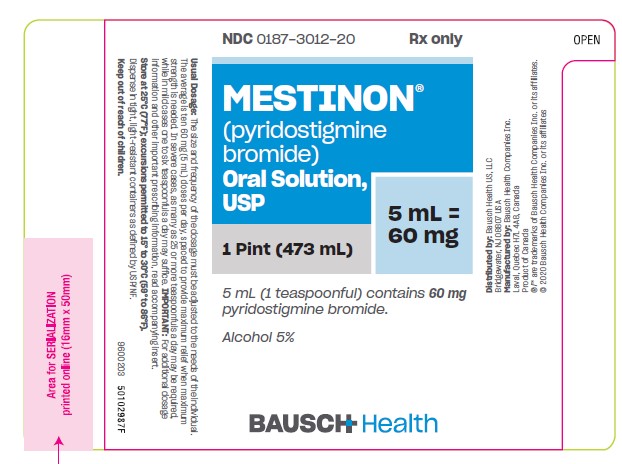
PRINCIPAL DISPLAY PANEL - 473 mL Bottle Label
NDC 0187-3012-20
Rx Only
MESTINON®
(pyridostigmine
bromide).
Oral Solution,
USP
1 Pint (473 mL)
5 mL =
60 mg
5 mL (1 teaspoonful) contains 60 mg
pyridostigmine bromide.
Alcohol 5%
BAUSCH Health
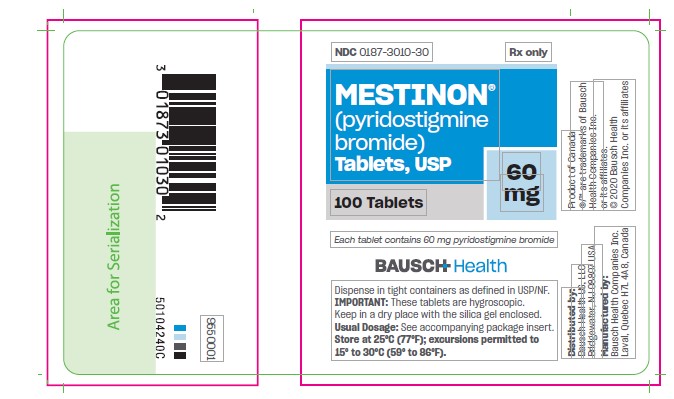
PRINCIPAL DISPLAY PANEL - 60 mg Tablets Bottle Label
NDC 0187-3010-30
Rx Only
Mestinon®
(pyridostigmine bromide)
Tablets, USP
60 mg
100 Tablets
Each tablet
contains 60 mg
pyridostigmine
bromide
BAUSCH Health
Dispense in tight containers as defined in USP/NF.
IMPORTANT: These tablets are hygrosciopic. Keep in dry place with the silica gel enclosed.
Usual Dosage: See accompanying package insert.
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F).