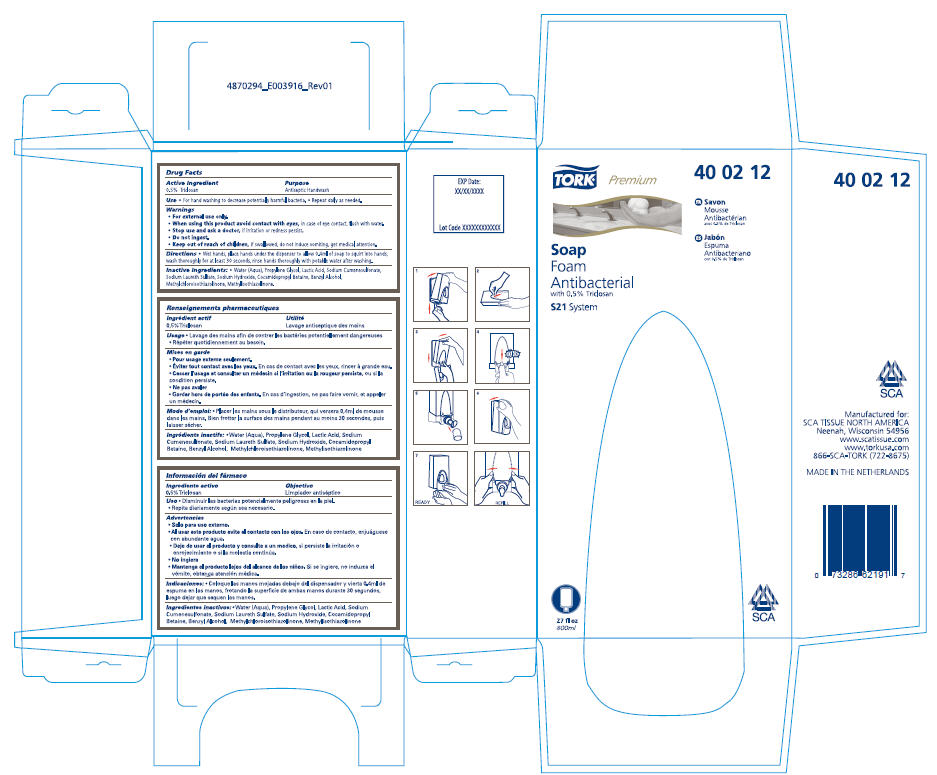
TORK PREMIUM- triclosan liquid
SCA Tissue North America
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
0.5% Triclosan
Purpose
Antiseptic Handwash
Use
- For hand washing to decrease potentially harmful bacteria.
- Repeat daily as needed.
Warnings
-
For external use only.
-
When using this product avoid contact with eyes, in case of eye contact, flush with water.
-
Stop use and ask a doctor, if irritation or redness persist.
-
Do not ingest.
-
Keep out of reach of children, if swallowed, do not induce vomiting, get medical attention.
Directions
- Wet hands, place hands under the dispenser to allow 0.4ml of soap to squirt into hands, wash thoroughly for at least 30 seconds, rinse hands thoroughly with potable water after washing.
Inactive Ingredients
- Water (Aqua), Propylene Glycol, Lactic Acid, Sodium Cumenesulfonate, Sodium Laureth Sulfate, Sodium Hydroxide, Cocamidopropyl Betaine, Benzyl Alcohol, Methylchloroisothiazolinone, Methylisothiazolinone.
Manufactured for:
SCA TISSUE NORTH AMERICA
Neenah, Wisconsin 54956
www.scatissue.com
www.torkusa.com
866-SCA-TORK (722-8675)
PRINCIPAL DISPLAY PANEL - 800 mL Carton
TORK®
Premium
40 02 12
Soap
Foam
Antibacterial
with 0.5% Triclosan
S21 System
FR Savon
Mousse
Antibactérian
avec 0.5% de Triclosan
ES Jabón
Espuma
Antibacteriano
con 0.5% de Triclosan
27 fl oz
800ml
SCA