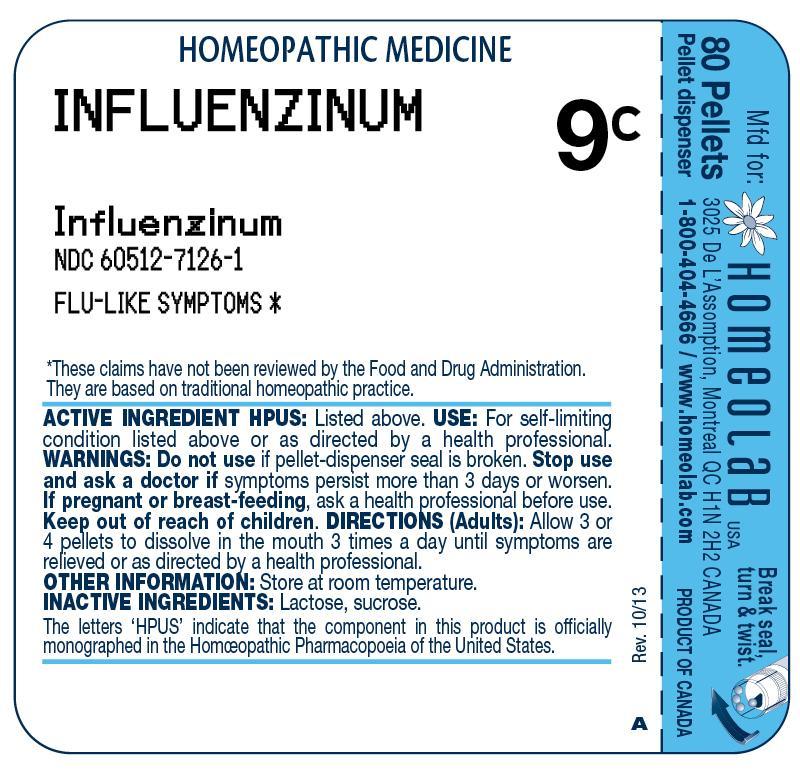
INFLUENZINUM- influenzinum pellet
HOMEOLAB USA INC.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENT HPUS
INFLUENZINUM 9C
(Influenzinum)
USE
For self-limiting condition listed above or as directed by a health professional.
WARNINGS
Do not use if pellet-dispenser seal is broken.
Stop use and ask a doctor if symptoms persist more than 3 days or worsen.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
DIRECTIONS
Adults: Allow 3 or 4 pellets to dissolve in the mouth 3 times a day until symptoms are relieved or as directed by a health professional.
OTHER INFORMATION
Store at room temperature.
INACTIVE INGREDIENTS
Lactose, sucrose.
QUESTIONS?
1-800-404-4666
The letters 'HPUS' indicate that the component in this product is officially monographed in the Homeopathic Pharmacopoeia of the United States.
These claims have not been reviewed by the Food and Drug Administration. They are based on traditional homeopathic practice.
80 Pellets
Pellet dispenser
Mfd for: HOMEOLAB USA INC., 3025 De L`Assomption, Montreal, QC, H1N 2H2, CANADA
Product of Canada
LABEL
HOMEOLAB USA INC.
