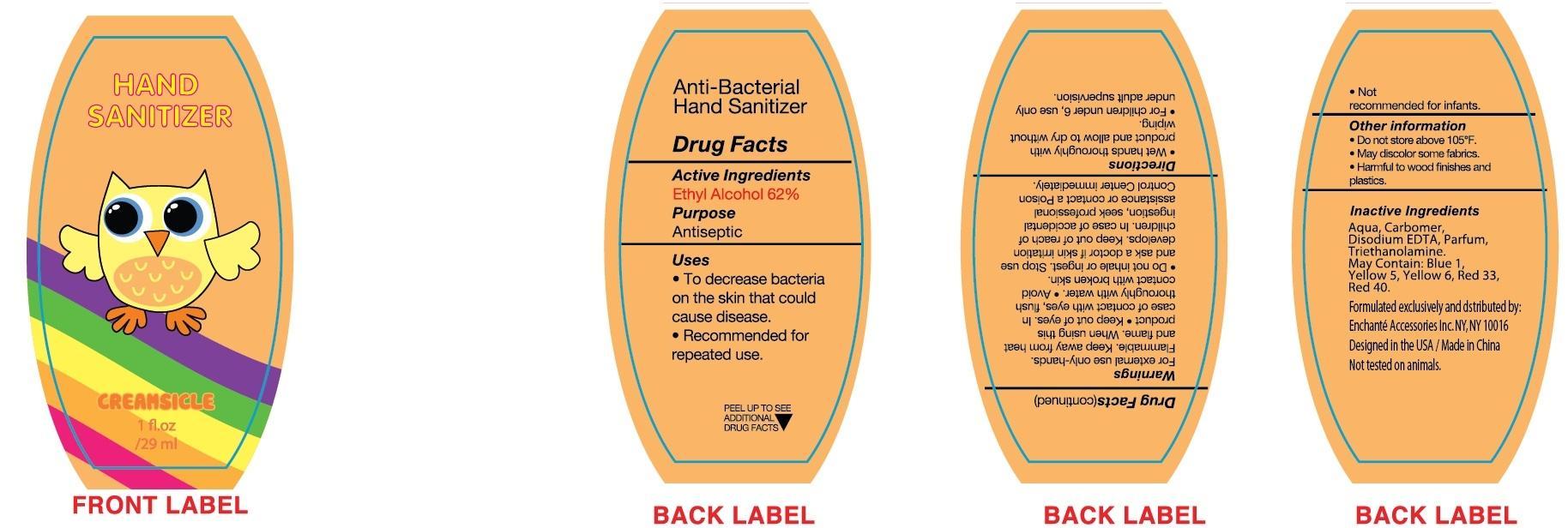
CREAMSICLE HAND SANITIZER- alcohol liquid
ENCHANTE ACCESSORIES INC.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active Ingredient
Ethyl Alcohol 62%
Uses
To decrease bacteria on the skin that could cause disease
Recommended for repeated use
For external use only-hands
Flammable. Keep away from heat and flame
Keep out of eyes. In case of comtact with eyes, flush thoroughly with water
Avoid contact with broken skin
Do not inhale or ingest. Stop use and ask a doctor if skin irritation develops
Keep out of reach of children.
In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Directions
- Wet hands thoroughly with product and allow to dry without wiping
- For children under 6, use only under adult supervision
- Not recommended for infants
Other Information
Do not store above 105F
May discolor some fabrics
Harmful to wood finishes and plastics
Inactive Ingredient:
Aqua, Carbomer, Triethanolamine, Parfum, Disodium EDTA, may contain D and C Red No. 33, FD and C Red No. 40, FD and C Blue No. 1, FD and C Yellow No. 5, FD and C Yellow No. 6.
ENCHANTE ACCESSORIES INC.