DESCRIPTION
Tobramycin ophthalmic solution USP, 0.3% is a sterile topical ophthalmic antibiotic formulation prepared specifically for topical therapy of external ophthalmic infections.
Each mL contains:
Active: tobramycin 3 mg (0.3%). Inactives: boric acid, sodium sulfate, sodium chloride, tyloxapol and purified water. Sodium hydroxide and/or sulfuric acid (to adjust pH). Tobramycin ophthalmic solution, 0.3% has a pH range between 7.0 and 8.0.
Preservative: benzalkonium chloride 0.1 mg (0.01%).
Tobramycin is a water-soluble aminoglycoside antibiotic active against a wide variety of gram-negative and gram-positive ophthalmic pathogens.
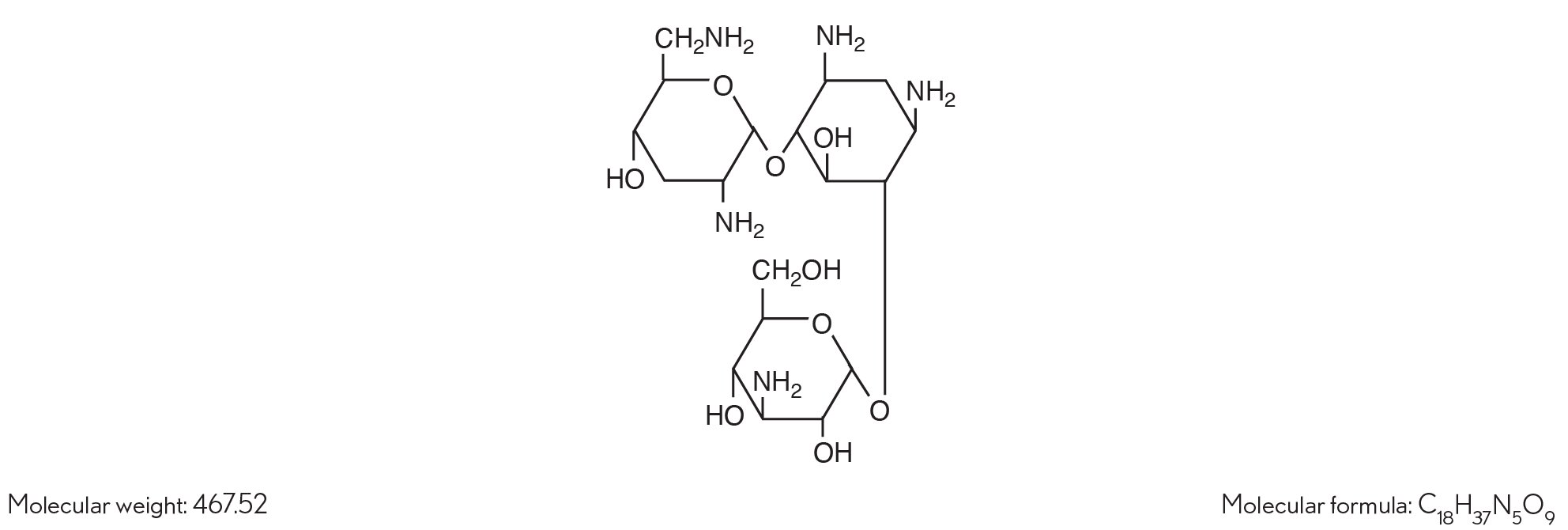
The chemical structure of tobramycin is:
Chemical name:
(2S,3R,4S,5S,6R)-4-amino-2-[(1S,2S,3R,4S,6R)-4,6-diamino-3-[(2R,3R,5S,6R)-3-amino-6-(aminomethyl)-5-hydroxyoxan-2-yl]oxy-2-hydroxycyclohexyl]oxy-6-(hydroxymethyl)oxane-3,5-diol
CLINICAL PHARMACOLOGY
In Vitro Data: In vitro studies have demonstrated tobramycin is active against susceptible strains of the following microorganisms: Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species. Bacterial susceptibility studies demonstrate that in some cases, microorganisms resistant to gentamicin retain susceptibility to tobramycin.
INDICATIONS AND USAGE
Tobramycin ophthalmic solution, 0.3% is a topical antibiotic indicated in the treatment of external infections of the eye and its adnexa caused by susceptible bacteria. Appropriate monitoring of bacterial response to topical antibiotic therapy should accompany the use of tobramycin ophthalmic solution. Clinical studies have shown tobramycin to be safe and effective for use in children.
CONTRAINDICATIONS
Tobramycin ophthalmic solution, 0.3% is contraindicated in patients with known hypersensitivity to any of its components.
WARNINGS
FOR TOPICAL OPHTHALMIC USE ONLY. NOT FOR INJECTION INTO THE EYE.
Sensitivity to topically applied aminoglycosides may occur in some patients. Severity of hypersensitivity reactions may vary from local effects to generalized reactions such as erythema, itching, urticaria, skin rash, anaphylaxis, anaphylactoid reactions, or bullous reactions. If a sensitivity reaction to tobramycin ophthalmic solution, 0.3% occurs, discontinue use.
PRECAUTIONS
General
As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be initiated.
Cross-sensitivity to other aminoglycoside antibiotics may occur; if hypersensitivity develops with this product, discontinue use and institute appropriate therapy. Patients should be advised not to wear contact lenses if they have signs and symptoms of bacterial ocular infection.
Information for Patients
Do not touch dropper tip to any surface, as this may contaminate the solution.
Pregnancy
Reproduction studies in 3 types of animals at doses up to 33 times the normal human systemic dose have revealed no evidence of impaired fertility or harm to the fetus due to tobramycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Because of the potential for adverse reactions in nursing infants from tobramycin ophthalmic solution, a decision should be made whether to discontinue nursing the infant or discontinue the drug, taking into account the importance of the drug to the mother.
ADVERSE REACTIONS
The most frequent adverse reactions to tobramycin ophthalmic solution, 0.3% are hypersensitivity and localized ocular toxicity, including lid itching and swelling, and conjunctival erythema. These reactions occur in less than three of 100 patients treated with tobramycin ophthalmic solution.
Postmarketing Experience
Additional adverse reactions identified from postmarketing use include anaphylactic reaction, Stevens-Johnson syndrome, and erythema multiforme.
The following additional adverse reactions have been reported with systemic aminoglycosides:
Neurotoxicity, ototoxicity and nephrotoxicity have occurred in patients receiving systemic aminoglycoside therapy. Aminoglycosides may aggravate muscle weakness in patients with known or suspected neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, because of their potential effect on neuromuscular function.
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE AND ADMINISTRATION
In mild to moderate disease, instill 1 or 2 drops into the affected eye(s) every 4 hours. In severe infections, instill 2 drops into the eye(s) hourly until improvement, following which treatment should be reduced prior to discontinuation.
FOR TOPICAL OPHTHALMIC USE ONLY
HOW SUPPLIED
Tobramycin ophthalmic solution USP, 0.3% is supplied in a plastic bottle with a controlled drop tip and a white polypropylene cap in the following size:
NDC 24208-290-05 - 5 mL fill in a 10 mL bottle
Storage
Store at 2°C to 25°C (36°F to 77°F). Avoid excessive heat.
After opening, tobramycin ophthalmic solution USP, 0.3% can be used until the expiration date on the bottle.
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Manufactured by:
Bausch & Lomb Incorporated
Tampa, FL 33637 USA
© 2023 Bausch & Lomb Incorporated or its affiliates
Revised: November 2023
9116808 (folded)
9116908 (flat)