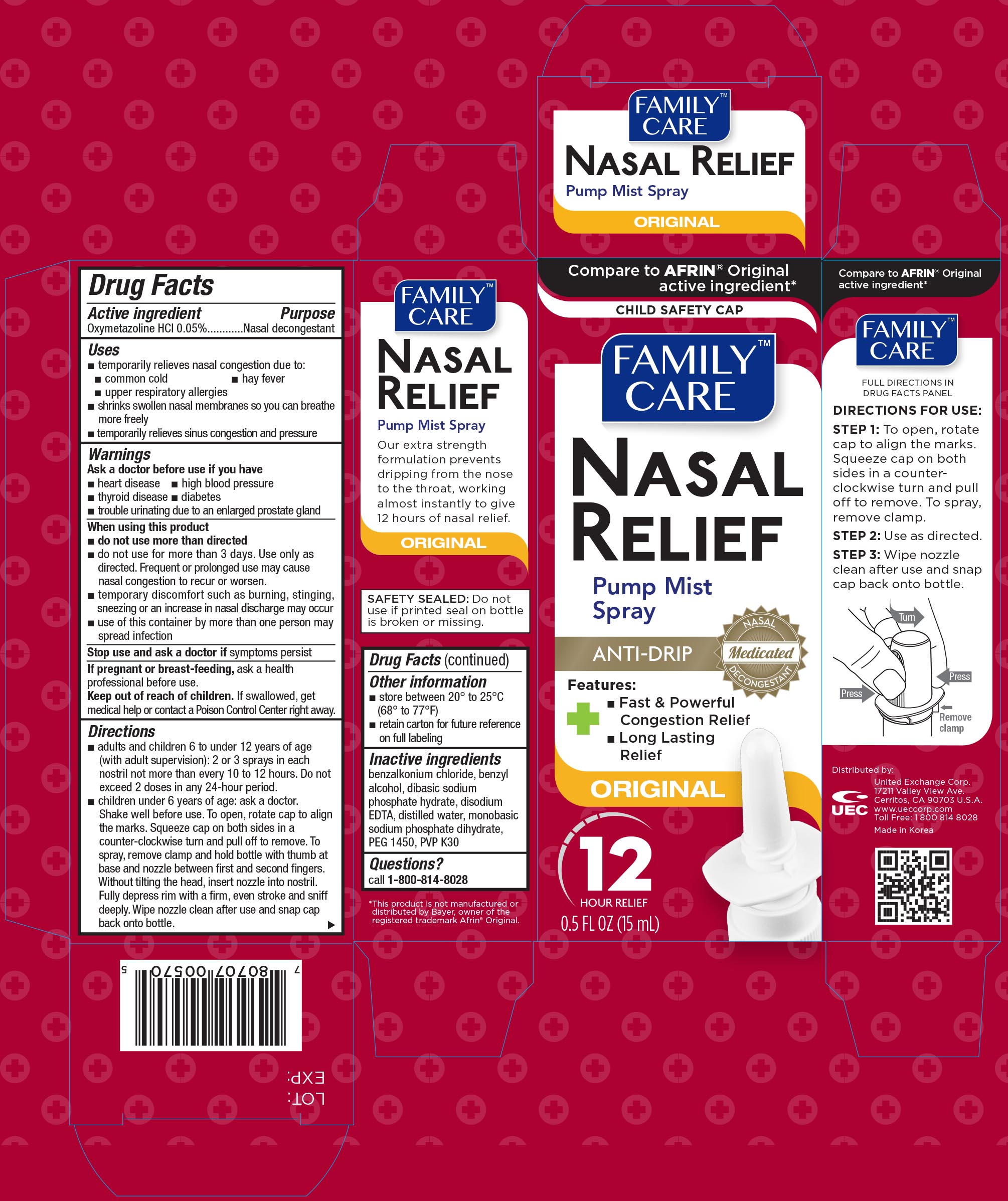
FAMILY CARE NASAL RELIEF- oxymetazoline hydrochloride spray
United Exchange Corp
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Family Care Nasal Spray Original 0.5 oz NBE Afrin Nasal Spray Original 570 (2018)
Active ingredient Purpose
Oxymetazoline HCl 0.05%.................................................Nasal Decongestant
Uses
- Temporarily relieves nasal congestion due to:
- common cold
- hay fever
- upper respiratory allergies
- shrinks swollen nasal membranes so you can breathe more freely
- temporarily relieves sinus congestion and pressure
Warnings
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
When using this product
- do not use more than directed
- do not use for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- use of this container by more than one person may spread infection
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 6 to 12 years of age (with adult supervision): 2 or 3 sprays in each nostril not more than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- children under 6 years of age: ask a doctor. Shake well before use. To open, rotate cap to align the marks. Squeeze cap on both sides in a counter-clockwise turn and pull off to remove. To spray, remove clamp and hold bottle with thumb at base and nozzle between first and second fingers. Without tilting the head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and sniff deeply. Wipe nozzle clean after use and snap cap back onto bottle.
Other information
- store between 20° and 25°C (68° and 77°F)
- retain carton for future reference on full labeling
| FAMILY CARE NASAL RELIEF
oxymetazoline hydrochloride spray |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - United Exchange Corp (840130579) |
Revised: 12/2021
Document Id: d3c4adb3-b0aa-77b3-e053-2a95a90a7e0d
Set id: a420da2c-5aa8-4398-acee-412c1dc346bb
Version: 7
Effective Time: 20211222
United Exchange Corp