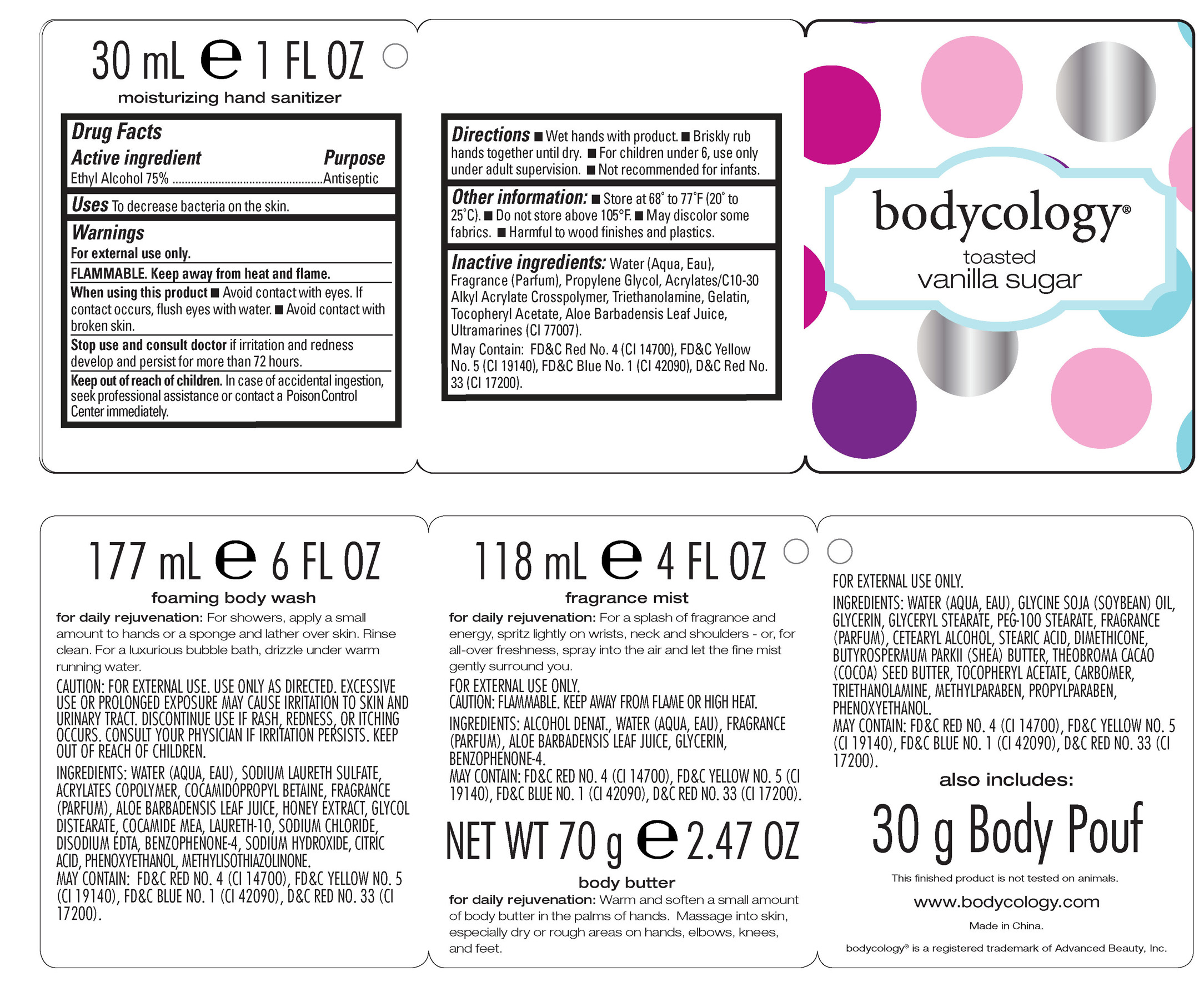
Drug Facts
Active ingredient Purpose
Ethyl Alcohol 75% Antiseptic
Uses To decrease bacteria on the skin.
Keep out of reach of children. In case of accidental ingestion, seek professional assistance or contact a Poison Control Center immediately.
Stop use and consult doctorif irritation and redness develop and persist for more than 72 hours.
Warnings
For external use only.
FLAMMABLE. Keep away from heat and flame.
When using this product
-Avoid contact with eyes. If contact occurs, flush eyes with water.
-Avoid contact with broken skin.

Directions
-Wet hands with product.
-Briskly rub hands together until dry.
-For children under 6, use only under adult supervision.
-Not recommended for infants.

Inactive ingredients: Water (Aqua, Eau), Fragrance (Parfum), Propylene Glycol, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Gelatin, Tocopheryl Acetate, Aloe Barbadensis Leaf Juice, Ultramarines (CI 77007).
May Contain: FD&C Red No. 4 (CI 14700), FD&C Yellow No. 5 (CI 19140), FD&C Blue No. 1 (CI 42090), D&C Red No. 33 (CI 17200).
