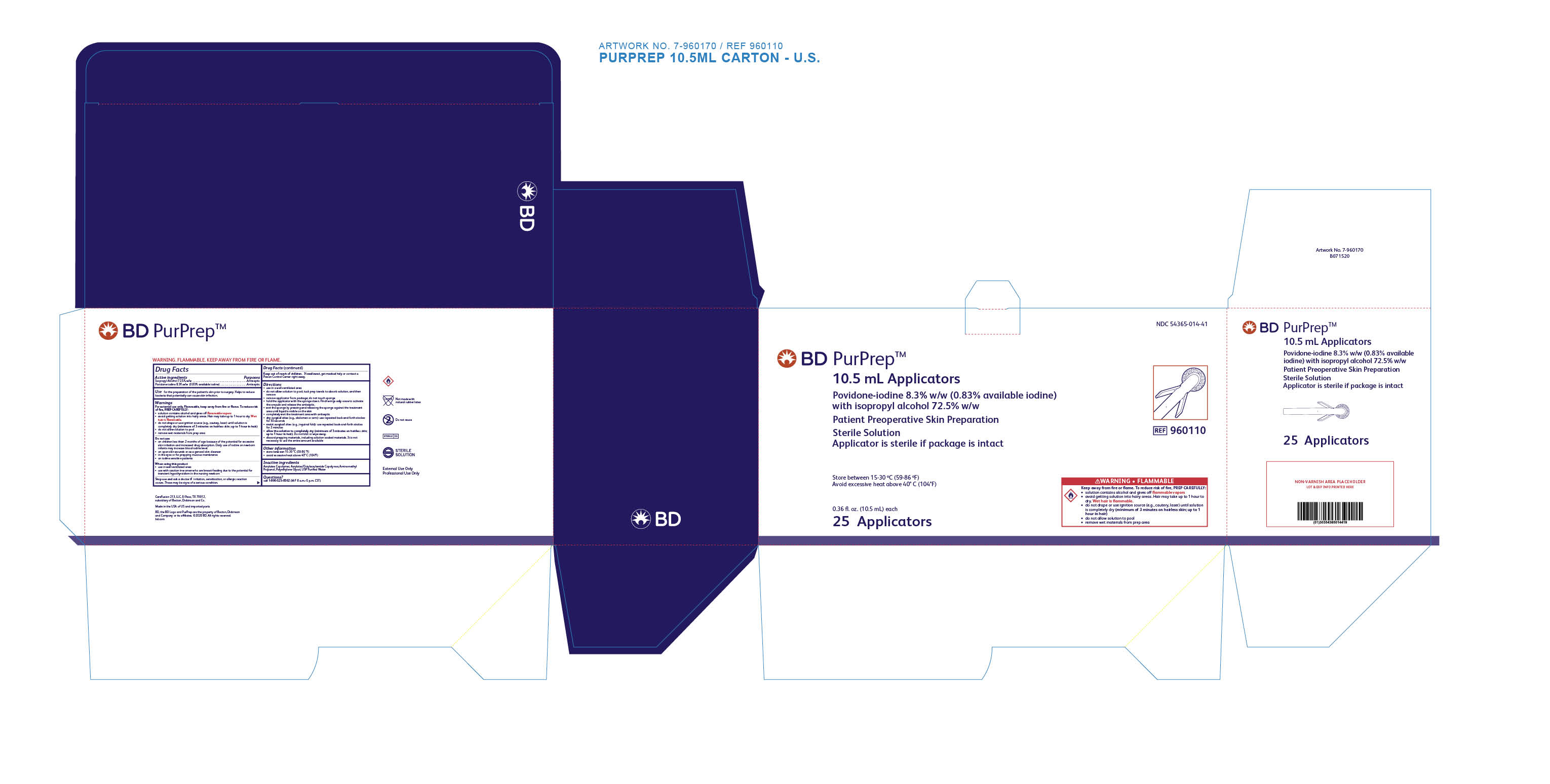
for the preparation of the patient's skin prior to surgery. Helps to reduce bacteria that potentially can cause skin infection.
For external use only. Flammable, keep away from fire or flame.
T o reduce risk of fire, PREP CAREFULLY:
- do not use 26-mL applicator for head and neck surgery
- solution contains alcohol and gives off flammable vapors.
- avoid getting solution into hairy areas. Hair may take up to 1 hour to dry. Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery, laser) until solution is completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair)
- do not allow the solution to pool
- remove wet materials from prep area
Do not use
- on children less than 2 months of age because of the potential for excessive skin irritation and increased drug absorption. Daily use of iodine on newborn infants may increase blood iodine level.
- on open skin wounds or as a general skin cleanser
- in the eyes or for prepping mucous membranes
- on iodine sensitive patients
When using this product
- use in well ventilated area.
- use with caution in women who are breast-feeding due to the potential for transient hypothyroidism in the nursing newborn
Stop use and ask a doctor if irritation, sensitization, or allergic reaction occurs. These maybe signs of a serious condition.
Keep out of the reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
-
- use in a well ventilated area
- do not allow solution to pool; tuck prep towels to absorb solution, and then remove
- remove applicator from package; do not touch sponge
- hold the applicator with the sponge down. Pinch wings only once to activate the ampules and release the antiseptic.
- wet the sponge by pressing and releasing the sponge against the treatment area until liquid is visible on the skin
- completely wet the treatment area with antiseptic
- dry surgical sites (e.g., abdomen or arm): use repeated back-and-forth strokes for 30 seconds
- moist surgical sites (e.g., inguinal fold): use repeated back-and-forth strokes for 2 minutes
- allow the solution to completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair). Do not blot or wipe away.
- discard prepping materials, including solution soaked materials. It is not necessary to use the entire amount available