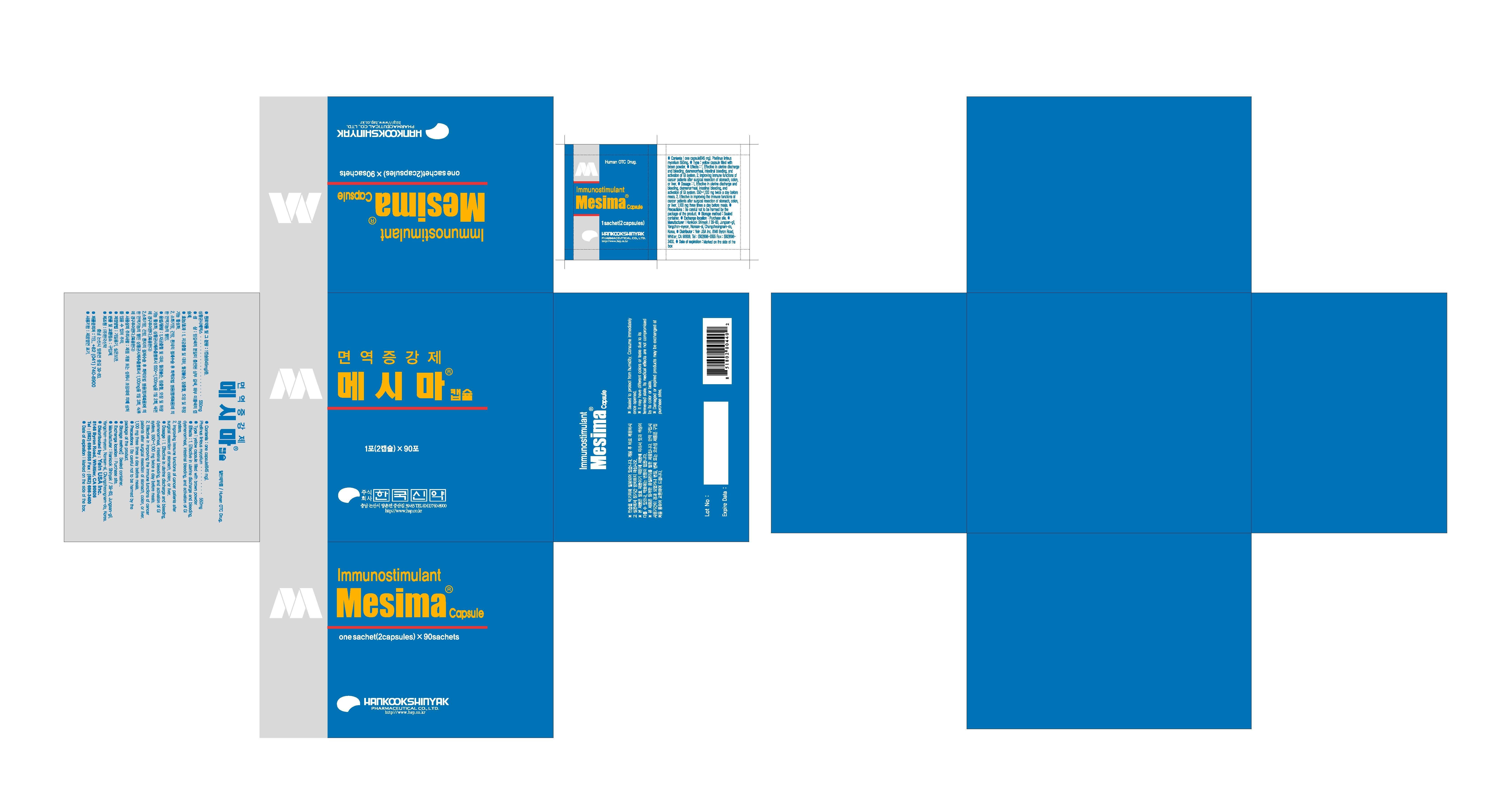
- effective in uterine discharge and bleeding, dysmenorrheal, intestinal bleeding and activation of GI system
-improving the immune functions of cancer patients after surgical resection of stomach, colon, or liver
-improving the immune functions of cancer patients after surgical resection of stomach, colon, or liver
- effective in uterine discharge and bleeding, dysmenorrheal, intestinal bleeding, and activation of GI system, 550-1100mg twice a day before meals
- effective in improving the immune functions of cancer patients after surgical resection of stomach, colon, or liver, 1100mg three times a day before meals
- effective in improving the immune functions of cancer patients after surgical resection of stomach, colon, or liver, 1100mg three times a day before meals