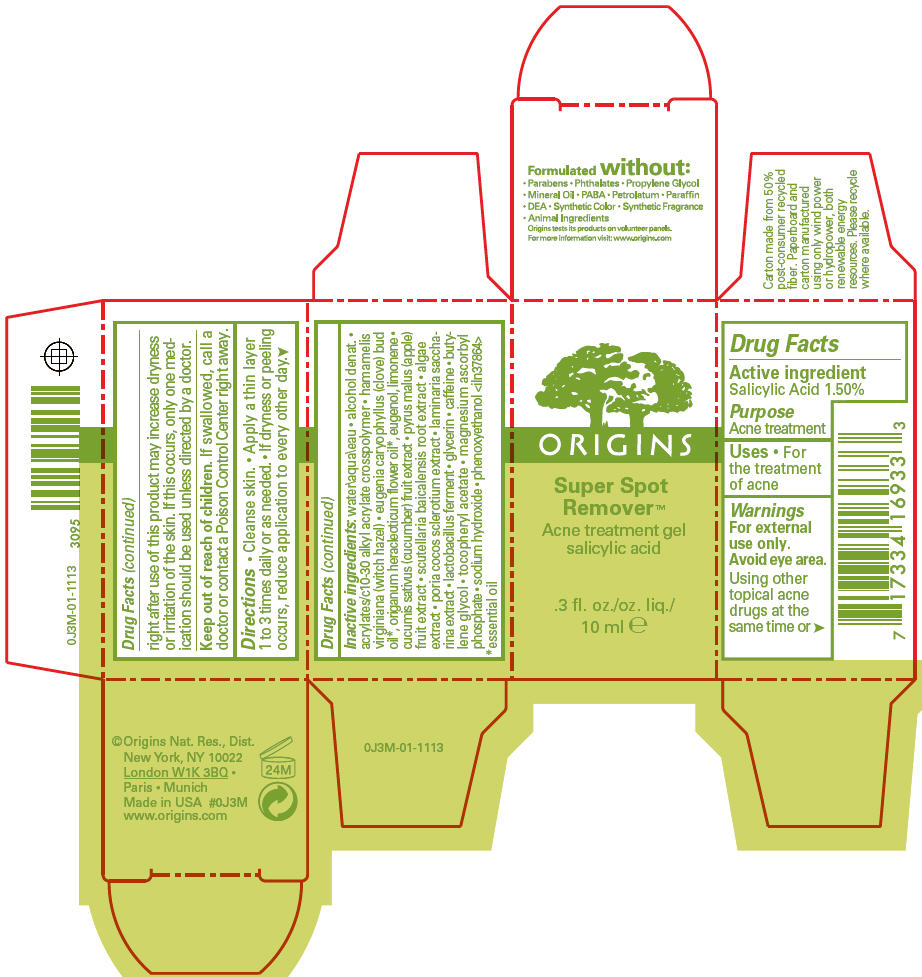
Warnings
For external use only.
Avoid eye area.
Using other topical acne drugs at the same time or right after use of this product may increase dryness or irritation of the skin. If this occurs, only one medication should be used unless directed by a doctor.
Directions
- Cleanse skin.
- Apply a thin layer 1 to 3 times daily or as needed.
- If dryness or peeling occurs, reduce application to every other day.
Inactive ingredients
water\aqua\eau • alcohol denat. • acrylates/c10-30 alkyl acrylate crosspolymer • hamamelis virginiana (witch hazel) • eugenia caryophyllus (clove) bud oil 1, origanum heracleoticum flower oil 1, eugenol, limonene • cucumis sativus (cucumber) fruit extract • pyrus malus (apple) fruit extract • scutellaria baicalensis root extract • algae extract • poria cocos sclerotium extract • laminaria saccharina extract • lactobacillus ferment • glycerin • caffeine • butylene glycol • tocopheryl acetate • magnesium ascorbyl phosphate • sodium hydroxide • phenoxyethanol <iln37864>
- 1
- essential oil