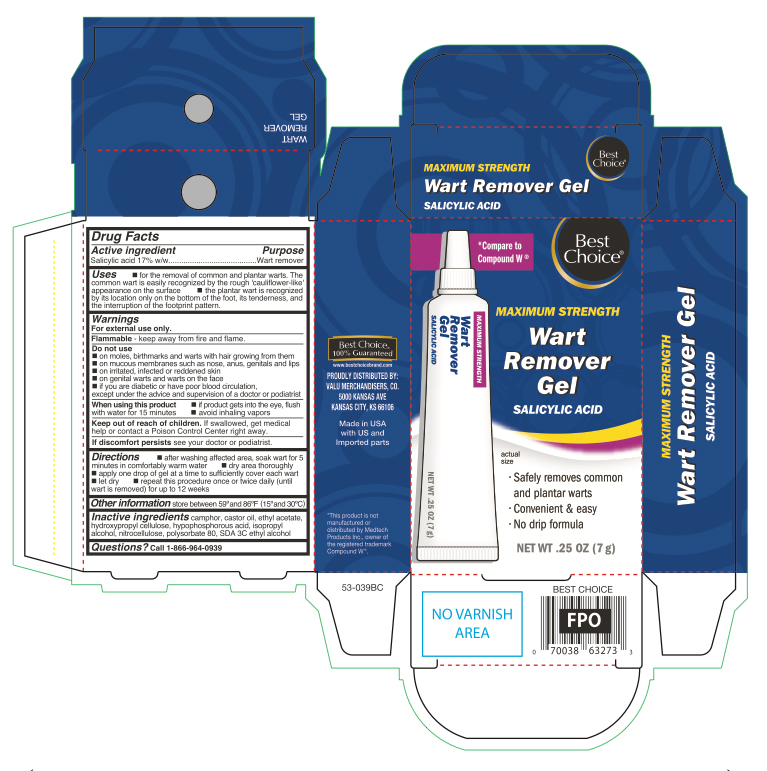
Uses
- for the removal of common and plantar warts. The common wart is easily recognized by the rough 'cauliflower-like' appearance of the surface.
- Plantar wart is recognized by its location only on the bottom of the foot, its tenderness and the interruption of the footprint pattern.
Warnings
For external use only.
Do not use
- on moles, birth marks and warts with hair growing from them
- on mucous membranes such as nose, anus, genitals and lips
- on irritated, infected or reddened skin
- on genital warts and warts on the face
- if you are diabetic or have poor blood circulation, except under the advice and supervision of a doctor or podiatrist
When using this product
- if product gets into the eyes, flush with water for 15 minutes
- avoid inhaling vapors
Directions
- after washing affected area, soak wart in for 5 minutes in comfortably warm water
- dry area thoroughly
- apply one drop of gel at a time to sufficiently cover each wart
- let dry
- repeat this procedure once or twice daily (until wart is removed) for up to 12 weeks