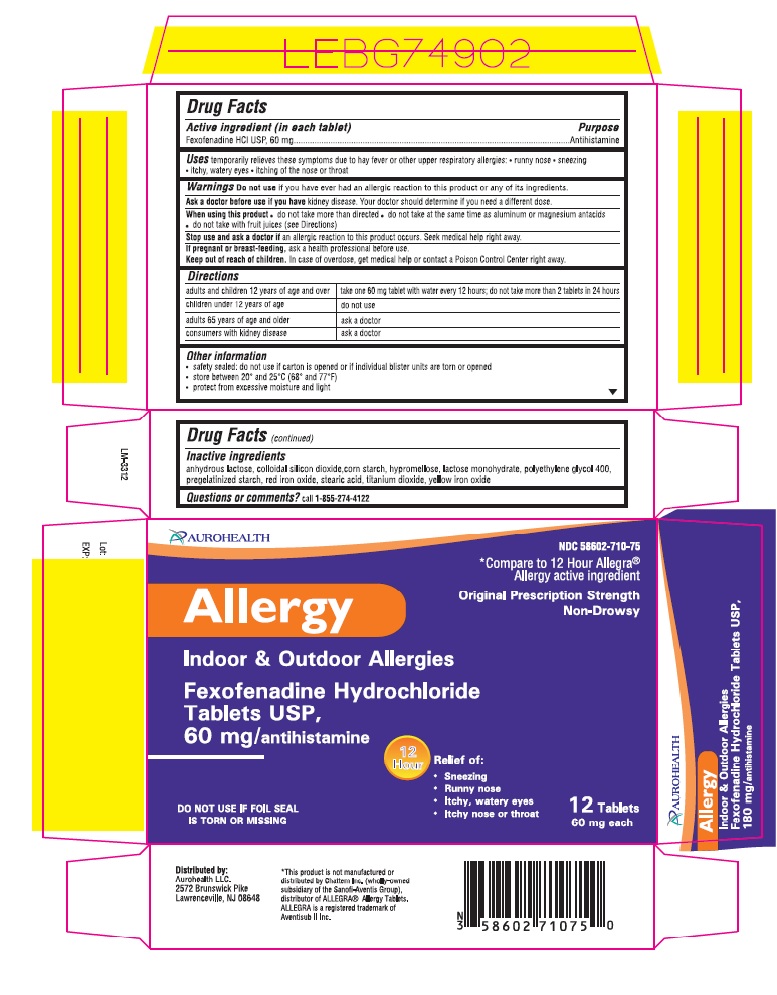
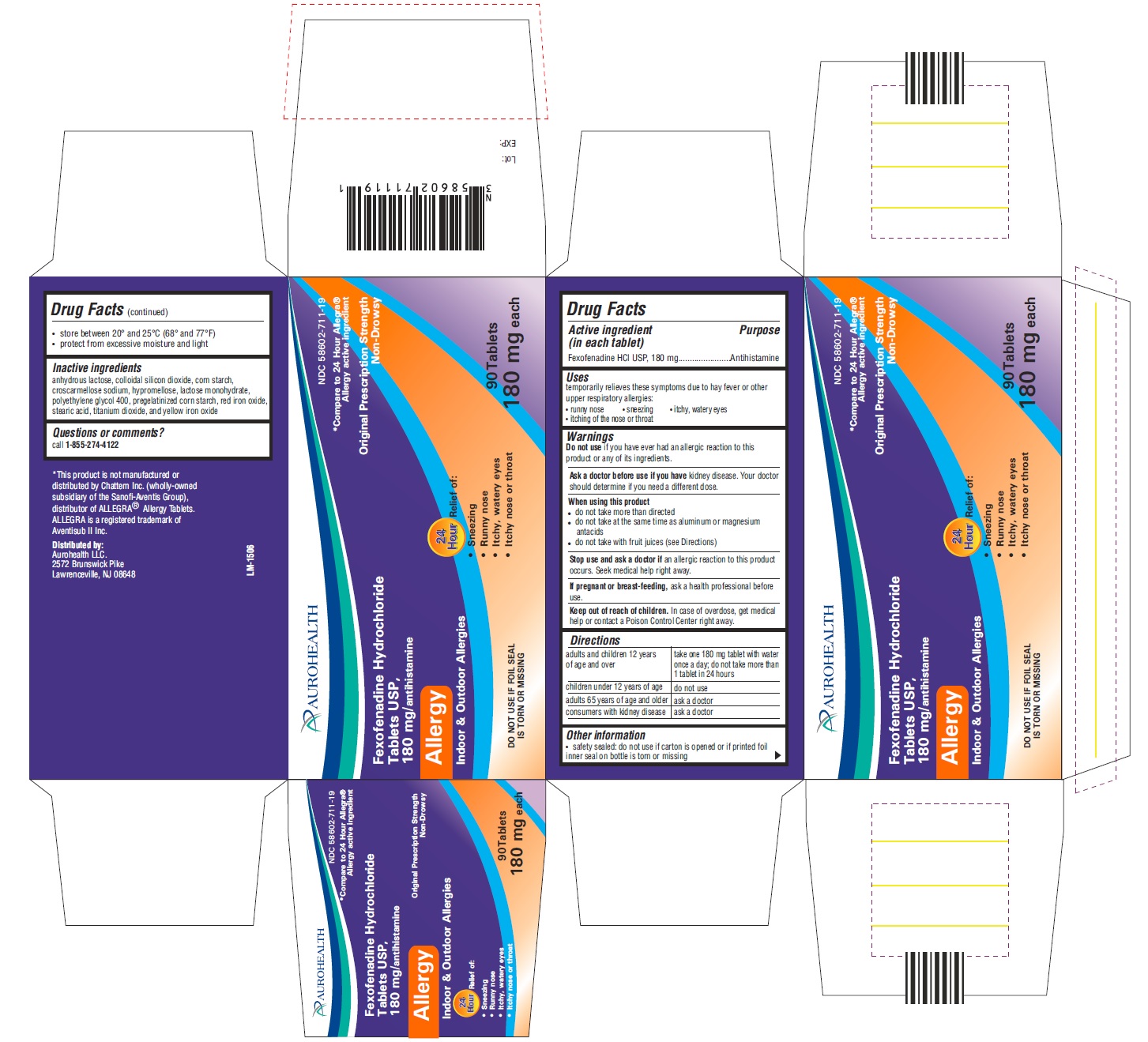
Uses
temporarily relieves these symptoms due to hay fever or other upper resporatory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminium or magnesium antacids
- do not take with fruit juices (see directions)
Stop use and ask doctor if
an allergic reaction to this product occurs. Seek medical help right away.
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years of age and over | take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
| adults and children 12 years of age and over | take one 60 mg tablet with water every 12 hours; do not take more than 2 tablet in 24 hours |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
- safety sealed: do not use if carton is opened or if printed foil inner seal on bottle is torn or missing
- store between 20°and 25°C (68°and 77°F)
- protect from excessive moisture and light
Inactive ingredients
anhydrous lactose, colloidal silicon dioxide, corn starch,croscarmellose sodium, hypromellose, lactose monohydrate, polyethylene glycol 400, pregelatinized corn starch, red iron oxide, steric acid, titanium dioxide, and yellow iron oxide,
Principal Display Panel
NDC 58602-711-19
*Compare to 24 Hour Allegra®
Allergy active ingrediant
Original Prescription Strength Non-Drowsy
Fexofenadine Hydrochloride Tablets USP, 180 mg/antihistamine
Allergy
Indoor & Outdoor Allergies
24 Hours Relief of:
Sneezing
Runny nose
Itchy, Watery Eyes
Itchy Nose or Throat
DO NOT USE IF FOIL SEAL IS TORN OR MISSING
90 Tablets 180 mg each
Principal Display Panel
NDC 58602-710-40
*Compare to 12 Hour Allegra®
Allergy active ingredient
Original Prescription Strength Non-Drowsy
Fexofenadine Hydrochloride Tablets USP, 60 mg/antihistamine
Allergy
Indoor & Outdoor Allergies
12 Hours Relief of:
Sneezing
Runny nose
Itchy, Watery Eyes
Itchy Nose or Throat
DO NOT USE IF FOIL SEAL IS TORN OR MISSING
500 Tablets 60 mg each

Principal Display Panel
NDC 58602-710-40
*Compare to 12 Hour Allegra®
Allergy active ingredient
Original Prescription Strength Non-Drowsy
Fexofenadine Hydrochloride Tablets USP, 60 mg/antihistamine
Allergy
Indoor & Outdoor Allergies
12 Hours Relief of:
Sneezing
Runny nose
Itchy, Watery Eyes
Itchy Nose or Throat
DO NOT USE IF FOIL SEAL IS TORN OR MISSING
12 Tablets 60 mg each