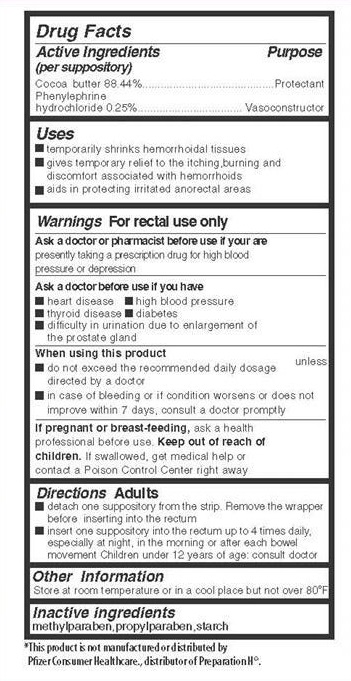
Active Ingredients
Active Ingredients
(per suppository)
Cocoa butter 88.44 %
Phenylephrine hydrochloride 0.25 %
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
- temporarily shrinks hemorrhoidal tissues
- gives temporary relief to the itching, burning and discomfort associated with hemorrhoids
- aids in protecting irritated anorectal areas
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- difficulty in urination due to enlargement of the prostate gland
When using this product
- do not exceed the recommended daily dosage unless directed by a doctor
- in case of bleeding or if condition worsens or does not improve within 7 days, consult a doctor promptly