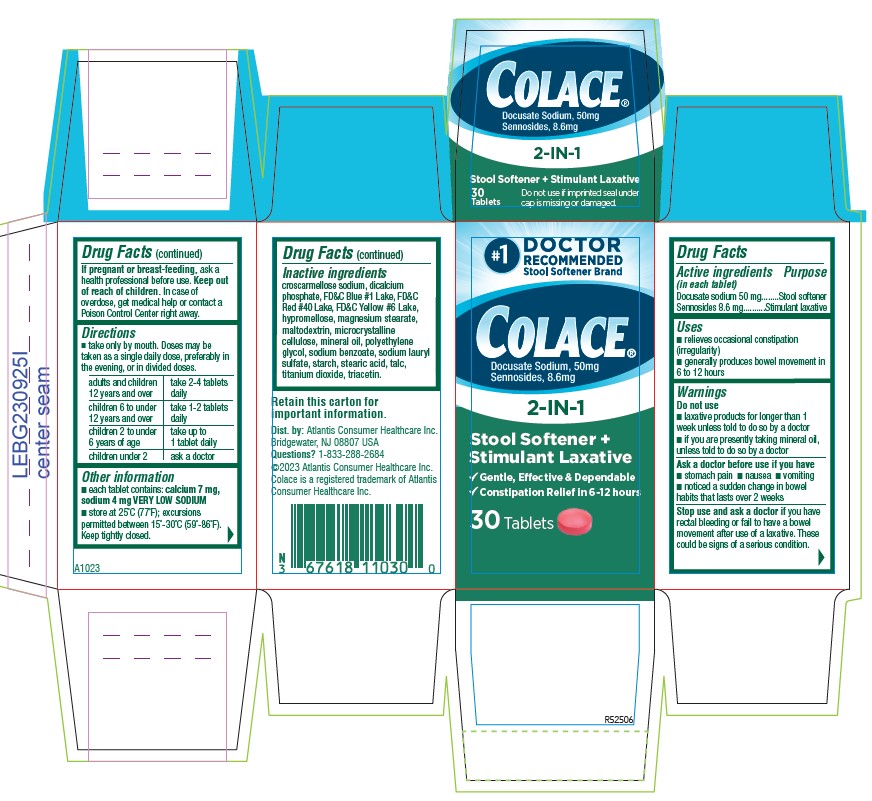
- laxative products for longer than 1 week unless told to do so by a doctor
- if you are presently taking mineral oil, unless told to do so by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that lasts over 2 weeks
Stop use and ask a doctor if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These could be signs of a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
- Take only by mouth. Doses may be taken as a single daily dose, preferably in the evening, or in divided doses.
| adults and children 12 years and over | take 2-4 tablets daily |
| children 6 to under 12 years of age | take 1-2 tablets daily |
| children 2 to under 6 years of age | take up to 1 tablet daily |
| children under 2 | ask a doctor |
- each tablet contains: calcium 7 mg, sodium 4 mg VERY LOW SODIUM
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F).
Keep tightly closed..
croscarmellose sodium, dicalcium phosphate, FD&C Blue #1 Lake, FD&C Red #40 Lake, FD&C Yellow #6 Lake, hypromellose, magnesium stearate, maltodextrin, microcrystalline cellulose, mineral oil, polyethylene glycol, sodium benzoate, sodium lauryl sulfate, starch, stearic acid, talc, titanium dioxide, triacetin.
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52504
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
#1
DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50 mg
Sennosides 8.6 mg
2-IN-1
Stool Softener +
Stimulant Laxative
√ Gentle, Effective, & Dependable
√ Constipation Relief in 6 `12 hours
10 Tablets

Colace® 2-IN-1 Tablets
Docusate Sodium
Sennosides
Dist. by: Atlantis Consumer
Healthcare Inc.
LOT XXXXXX
EXP YYYY-MM

#1
DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50 mg
Sennosides 8.6 mg
2-IN-1
Stool Softener +
Stimulant Laxative
√ Gentle, Effective, & Dependable
√ Constipation Relief in 6 `12 hours
30 Tablets
Retain this carton for
Important information
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52506
Colace®
Docusate Sodium
Sennosides
2-IN-1
Stool Softener +
Stimulant Laxative
30 Tablets
Do not use if imprinted seal under cap
is missing or damaged.
See carton for additional information
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52507

#1
DOCTOR
RECOMMENDED
Stool Softener Brand
Colace®
Docusate Sodium, 50 mg
Sennosides 8.6 mg
2-IN-1
Stool Softener +
Stimulant Laxative
√ Gentle, Effective, & Dependable
√ Constipation Relief in 6 `12 hours
Retain this carton for
Important information
For added savings, visit:
colacecapsules.com
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
A1023
R52508
60 Tablets
A1023
R52507

Colace®
Docusate Sodium
Sennosides
2-IN-1
Stool Softener +
Stimulant Laxative
60 Tablets
Do not use if imprinted seal under cap
is missing or damaged.
See carton for additional information
Dist. by: Atlantis Consumer Healthcare inc.
Bridgewater, NJ 08801 USA
Questions? 1-833-286-2684
©2023 Atlantis Consumer Healthcare inc.
Colace is a registered trademark of Atlantis
Consumer Healthcare Inc.
LOT:
EXP:
A1023
R52509
