VARICOSE VEIN COMPLEX - aesculus hippocastanum, arnica montana, berberis vulgaris, carbo vegetabilis, echinacea angustifolia, hamamelis virginiana, hydrofluoricum acidum, lycopodium clavatum, secale cornutum, sulfur liquid
Nova Homeopathic Therapeutics, Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Purpose:
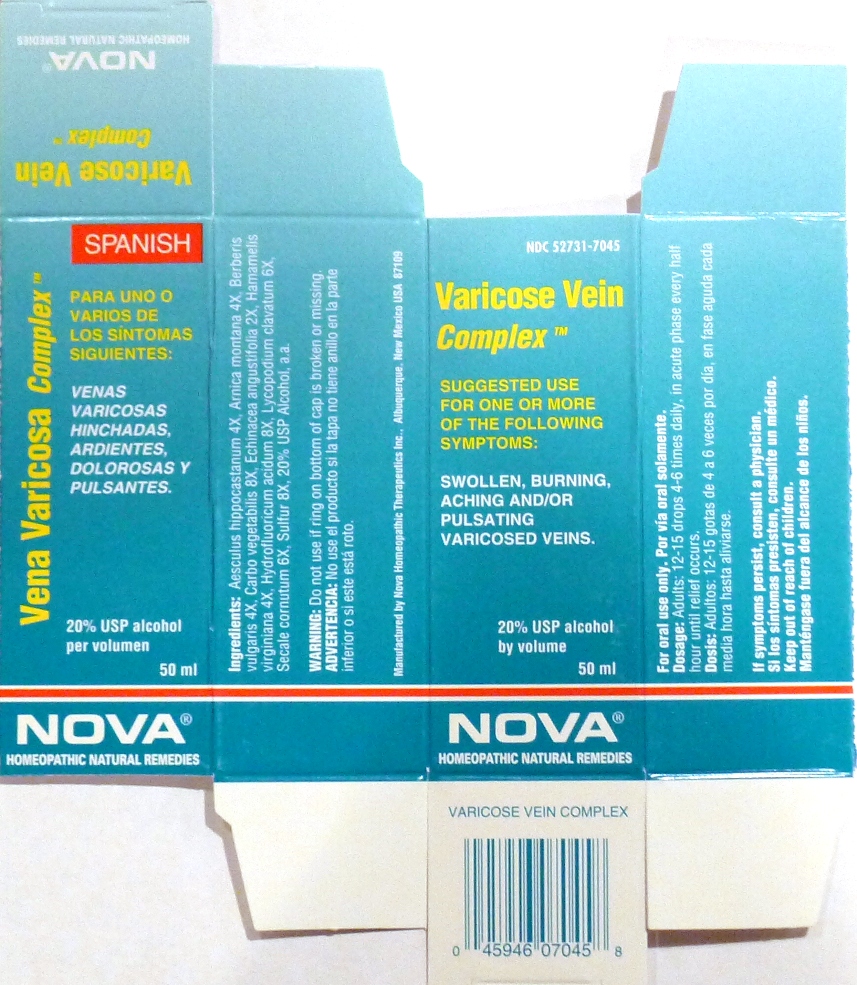
Suggested use for one or more of the following symptoms:
Swollen, burning, aching and/or pulsating varicosed veins.
Usage and Dosage:
For oral use only.
Adults:
In Acute Phase:
12-15 drops, every half hour until relief occurs
When Relief Occurs:
12-15 drops, 4-6 times per day
Warnings:
Keep out of reach of children.
If symptoms persist, consult a physician.
Do not use if ring on bottom of cap is broken or missing.
Active Ingredients:
Aesculus hippocastanum 4X, Arnica montana 4X, Berberis vulgaris 4X, Carbo vegetabilis 8X, Echinacea angustifolia 2X, Hamamelis virginiana 4X, Hydrofluoricum acidum 8X, Lycopodium clavatum 6X, Secale cornutum 6X, Sulfur 8X
Inactive Ingredients:
Alcohol, 20% USP
Manufactured by Nova Homeopathic Therapeutics Inc., Albuquerque, New Mexico USA 87109
1-800-225-8094
Varicose Vein Complex Product
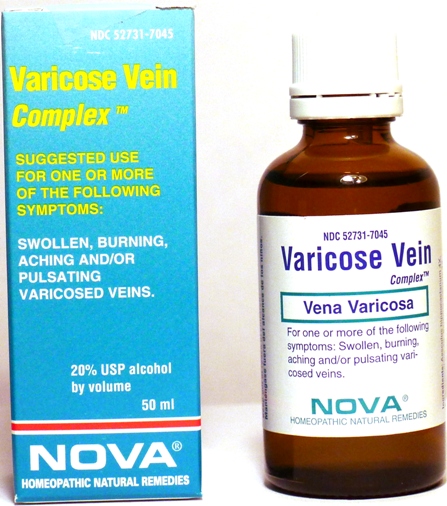
Varicose Vein Complex Bottle Label

Varicose Vein Complex Box
Nova Homeopathic Therapeutics, Inc.


