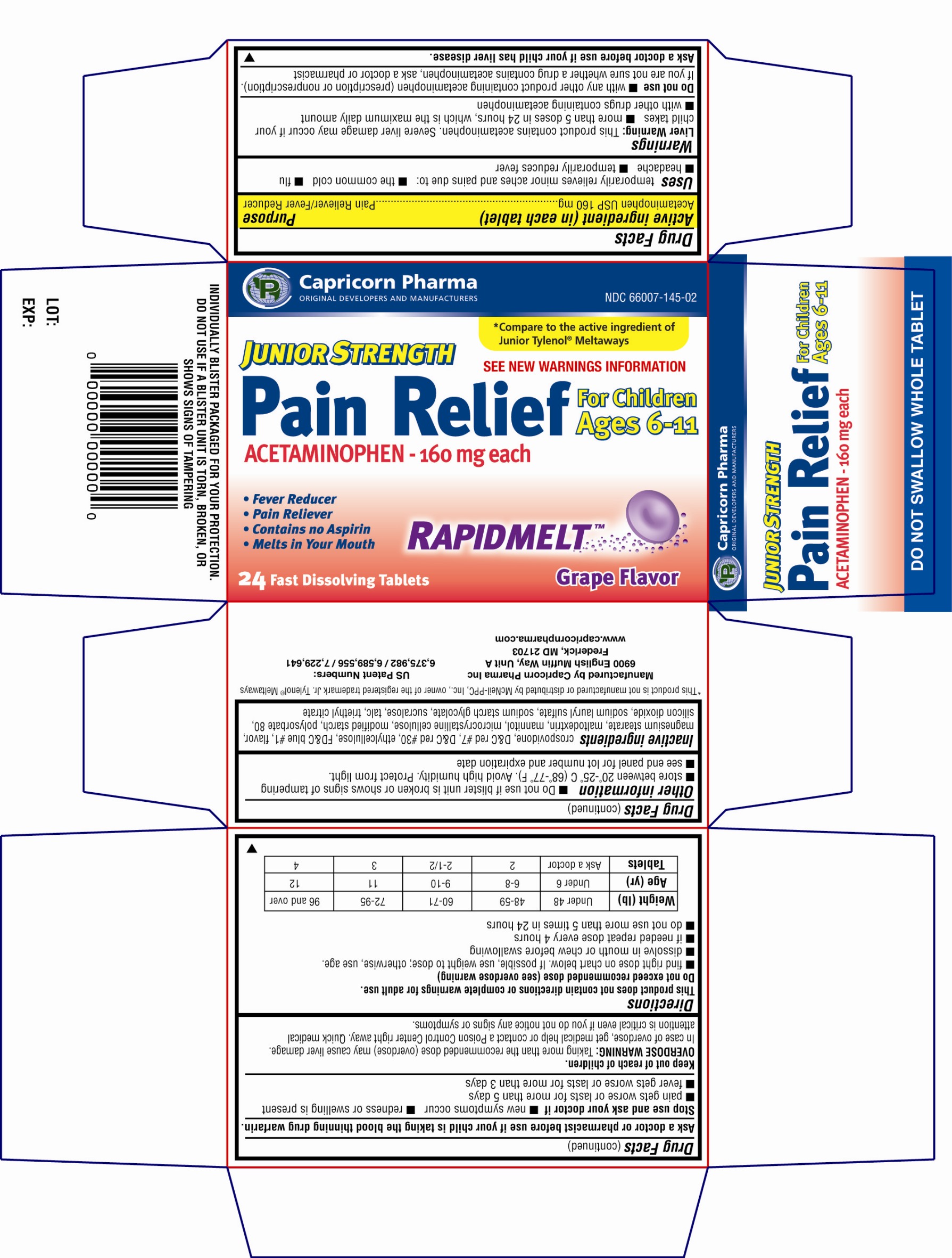
Uses
temporarily relieves minor aches and pains due to:
■ the common cold
■ flu
■ headache
■ temporarily reduces fever
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
■ more than 5 doses in 24 hours, which is maximum daily amount
■ with other drugs containing acetaminophen
Do not use
■ with any other product containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Directions
This product does not contain directions or complete warnings for adult use.
Do not exceed recommended dose (see overdose warning)
■ find right dose on chart below. If possible, use weight to dose; otherwise, use age
■ dissolve in mouth or chew before swallowing
■ if needed, repeat dose every 4 hours
■ do not use more than 5 times in 24 hours
| Weight (lb) | Under 48 | 48-59 | 60-71 | 72-95 | 96 and over |
| Age (yr) | Under 6 | 6-8 | 9-10 | 11 | 12 |
| Tablets | Ask a doctor | 2 | 2-1/2 | 3 | 4 |
Other Information
■ Do not use if blister unit is broken or shows signs of tampering.
■ Store between 20-25ºC (68-77ºF). Avoid high humidity. Protect from light.
■ See end panel for lot number and expiration date.
Inactive Ingredient
Crospovidone, D & C red # 7, D & C red # 30, ethylcellulose, FD & C blue # 1, flavor, magnesium stearate, maltodextrin, mannitol, microcrystalline cellulose, modified starch, polysorbate 80, silicon dioxide, sodium lauryl sulfate, sodium starch glycolate, sucralose, talc, triethyl citrate
Principal Display Panel
Capricorn PharmaORIGINAL DEVELOPERS AND MANUFACTURERS
Compare to the active ingredient of Junior Tylenol Meltaways
JUNIOR STRENGTH
Pain Relief
ACETAMINOPHEN - 160mg each
For Children Ages 6-11
See new warnings information
* Fever Reducer
* Pain Reliever
* Contains no Aspirin
* Melts in your mouth
RAPIDMELT
24 Fast Dissolving Tablets
Grape Flavor