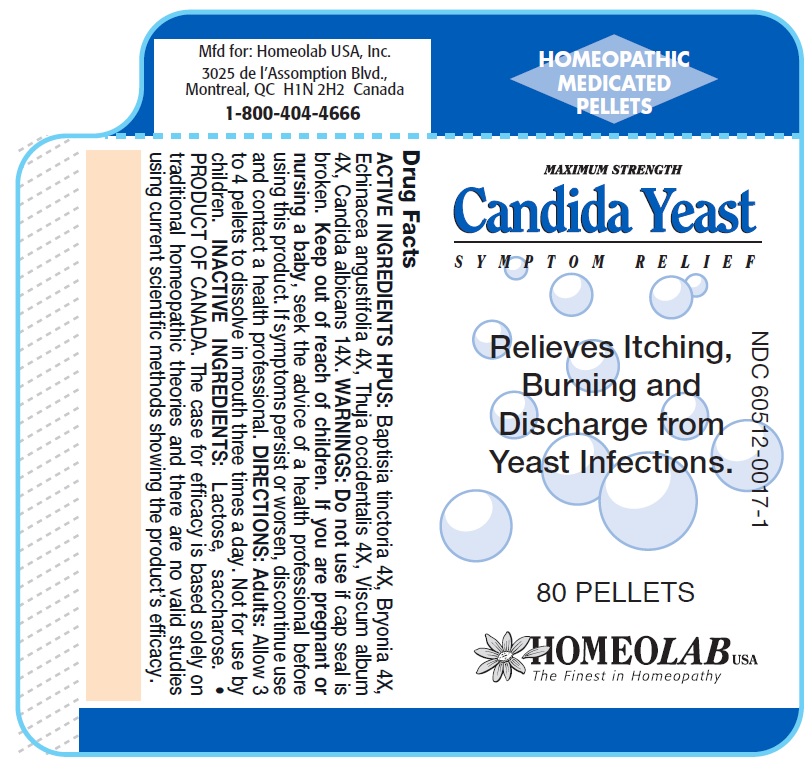
CANDIDA YEAST RELIEF- baptisia tinctoria, bryonia alba, echinacea angustifolia, thuja occidentalis, viscum album, candida albicans pellet
HOMEOLAB USA INC.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS HPUS
Baptisia tinctoria 4X
Bryonia alba 4X
Echinacea angustifolia 4X
Thuja occidentalis 4X
Viscum album 4X
Candida albicans 14X
Manufactured according to the Homeopathic Pharmacopoeia of the United States (HPUS)
PURPOSE
Relief of Itching, Burning and Discharge from Yeast Infections
USES
This is a natural Homeopathic remedy that relieves itching, burning and discharge from yeast infections.
If symptoms persist or worsen, discontinue use and contact a health professional.
As with any drug, if you are pregnant or nursing a baby, seek the advice of a health professional before using this product.
Keep this and all medications out of reach of children.
DIRECTIONS
Adults: Allow 3 or 4 pellets to dissolve in mouth three times a day.
Not for use by children.
OTHER INFORMATION
Do not use if cap seal is broken.
Homeolab USA Inc., 3025 De L'Assomption Blvd., Montreal, QC H1N 2H2 Canada
PRODUCT OF CANADA
Package Labeling:

HOMEOLAB USA INC.

