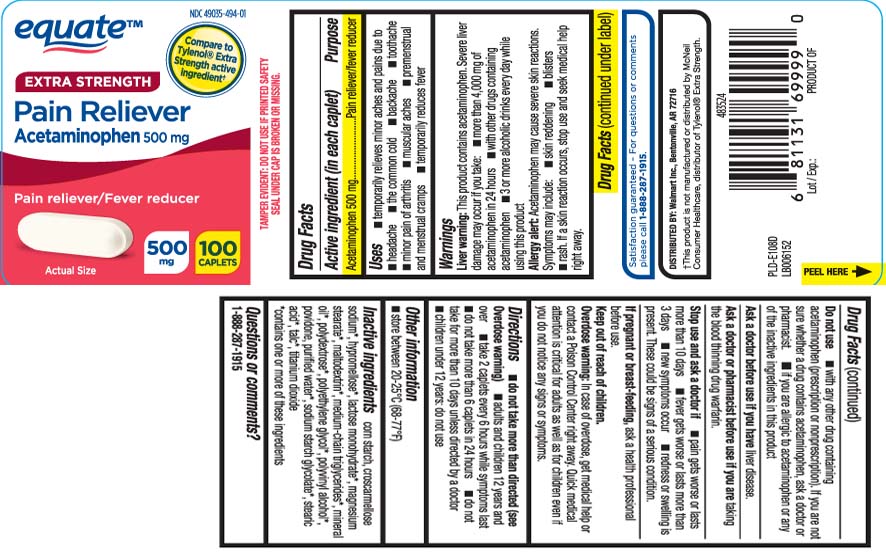
Uses
- temporarily relieves minor aches and pains due to
- headache
- the common cold
- backache
- minor arthritis pain
- toothache
- muscular aches
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks ever day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are allergic to acetaminophen or any of the inactive ingredients in this product
Directions
- do not take more than directed (see Overdose warning)
- adults and children 12 years and over
- take 2 caplets every 6 hours while symptoms last
- do not take more than 6 caplets in 24 hours
- do not take for more than 10 days unless directed by a doctor
- children under 12 years: do not use
Inactive ingredients
corn starch, croscarmellose sodium*, hypromellose*, lactose monohydrate*, magnesium stearate*, maltodextrin*, medium-chain triglycerides*, mineral oil*, polydextrose*, polyethylene glycol*, polyvinyl alcohol*, povidone, purified water*, sodium starch glycolate*, stearic acid*, talc*, titanium dioxide
*contains one or more of these ingredients
Principal Display Panel
Compare to Tylenol® Extra Strength Active Ingredient†
EXTRA STRENGTH
Pain Reliever
Acetaminophen 500 mg
Pain reliever/Fever reducer
CAPLETS
TAMPER EVIDENT: DO NOT USE IF PRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
†This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Tylenol® Extra Strength.
DISTRIBUTED BY:
Wal-Mart Inc.,
Bentonville, AR 72716