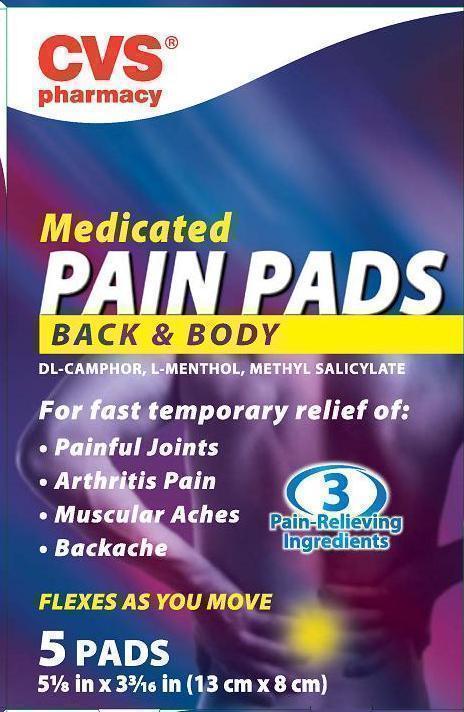
MEDICATED PAIN
- camphor (synthetic), levomenthol and methyl salicylate patch
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
Purpose
dl-Camphor External analgesic
l-Menthol External analgesic
Methyl Salicylate External analgesic
Uses temporary relieves minor aches and pains of muscles and joints associated with
■ simple backache ■ arthritis ■ strains ■ bruises ■ sprains
Warnings
For external use only
Directions
■ Strip off the polyethylene film and place adhesive pad over affected area.
■ Change pad 1 or 2 times a day
■ Not for children under 4 years.
Inactive ingredients
aluminum hydroxide gel, carbomer, carmellose sodium, edetate disodium, glycerin, malic acid, methacrylic acid and n-butyl acrylate copolymer, partially hydrolyzed polyvinyl alcohol, partially neutralized polyacrylate, PEG 60 hydrogenated castor oil, sorbitol, titanium dioxide, water.
| MEDICATED PAIN
dl-camphor, l-menthol, methyl salicylate patch |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - CVS Pharmacy (062312574) |
| Registrant - Sato Pharmaceutical Co., Ltd. (690575642) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fuji Seal, Inc. | 718023935 | label(59779-606) , pack(59779-606) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sato Pharmaceutical Co., Ltd. | 715699133 | manufacture(59779-606) | |