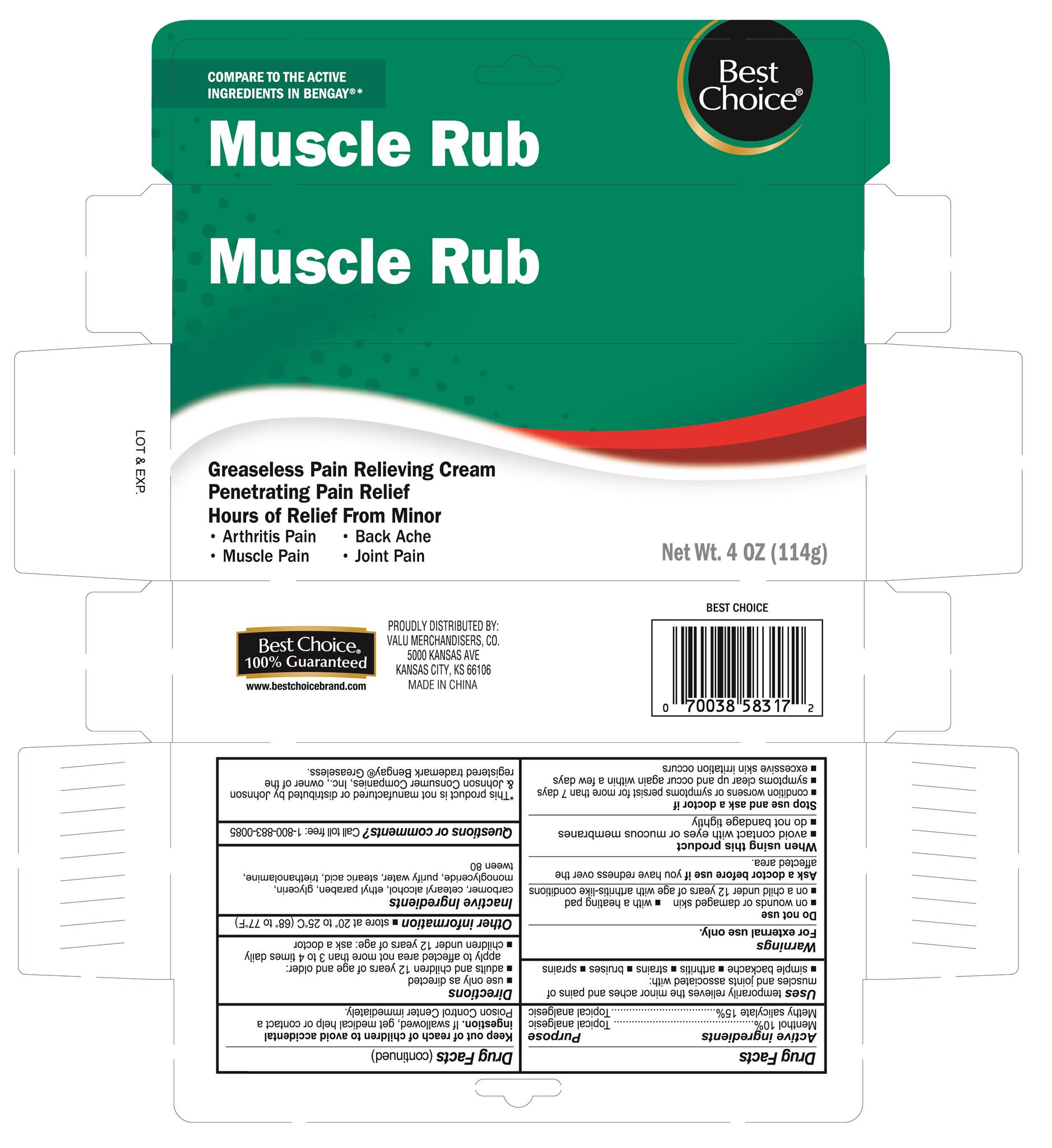
MUSCLE AND JOINT PAIN RELIEF- menthol, methyl salicylate cream
Valu Merchandisers, Co.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Active ingredients
Menthol 10%
Methyl salicylate 15%
Uses
temporarily relieves the minor aches and pains of muscles and joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
Do not use
- on wounds or damaged skin
- with a heating pad
- on a child under 12 years of age with arthritis-like conditions
Ask a doctor before use if you have
redness over the affected area.
When using this product
- avoid contact with eyes or mucous membranes
- do not bandage tightly
Stop use and ask a doctor if
- condition worsens or symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive skin irritation occurs
Keep out of reach of children to avoid accidental ingestion.
If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
- use only as directed
- adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 12 years of age: ask a doctor
Other information
- store at 20° to 25°C (68° to 77°F)
Inactive ingredients
carbomer, cetearyl alcohol, ethyl paraben, glycerin, monoglceride, purify water, stearic acid, triethanolamine, tween 80
Purpose
Topical analgesic
Topical analgesic
Package label
Best Choice Greaseless Muscle Rub
Valu Merchandisers, Co.
