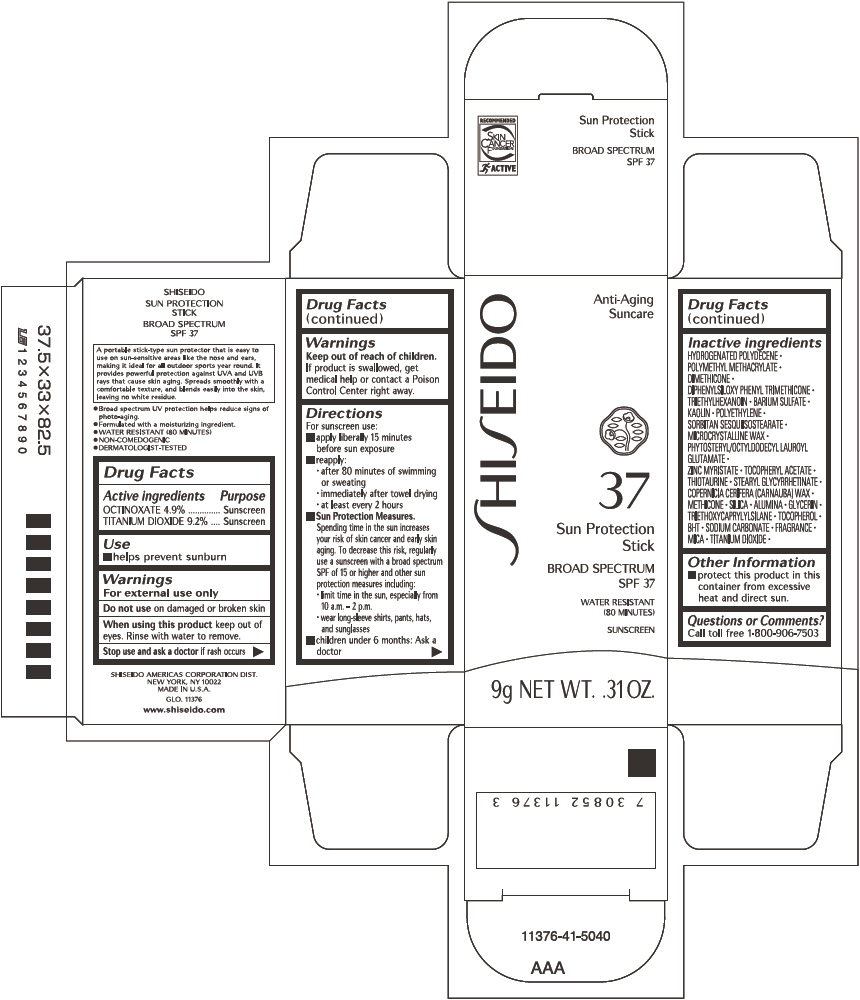
SHISEIDO SUN PROTECTION- octinoxate and titanium dioxide stick
SHISEIDO AMERICAS CORPORATION
----------
SHISEIDO SUN PROTECTION
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply:
- after 80 minutes of swimming or sweating
- immediately after towel drying
- at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
Inactive Ingredients
HYDROGENATED POLYDECENE, POLYMETHYL METHACRYLATE, DIMETHICONE, DIPHENYLSILOXY PHENYL TRIMETHICONE, TRIETHYLHEXANOIN, BARIUM SULFATE, KAOLIN, POLYETHYLENE, SORBITAN SESQUIISOSTEARATE, MICROCRYSTALLINE WAX, PHYTOSTERYL/OCTYLDODECYL LAUROYL GLUTAMATE, ZINC MYRISTATE, TOCOPHERYL ACETATE, THIOTAURINE, STEARYL GLYCYRRHETINATE, COPERNICIA CERIFERA (CARNAUBA) WAX, METHICONE, SILICA, ALUMINA, GLYCERIN, TRIETHOXYCAPRYLYLSILANE, TOCOPHEROL, BHT, SODIUM CARBONATE, FRAGRANCE, MICA, TITANIUM DIOXIDE,
| SHISEIDO SUN PROTECTION
octinoxate and titanium dioxide stick |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - SHISEIDO AMERICAS CORPORATION (193691821) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| SHISEIDO AMERICA INC. | 782677132 | manufacture(58411-161) , analysis(58411-161) | |
Revised: 10/2023
Document Id: f8d5910e-6c6a-4738-8eb0-d74f77ef4b26
Set id: 9b3a0a99-b701-420b-8d45-cdcae332e141
Version: 3
Effective Time: 20231022
SHISEIDO AMERICAS CORPORATION