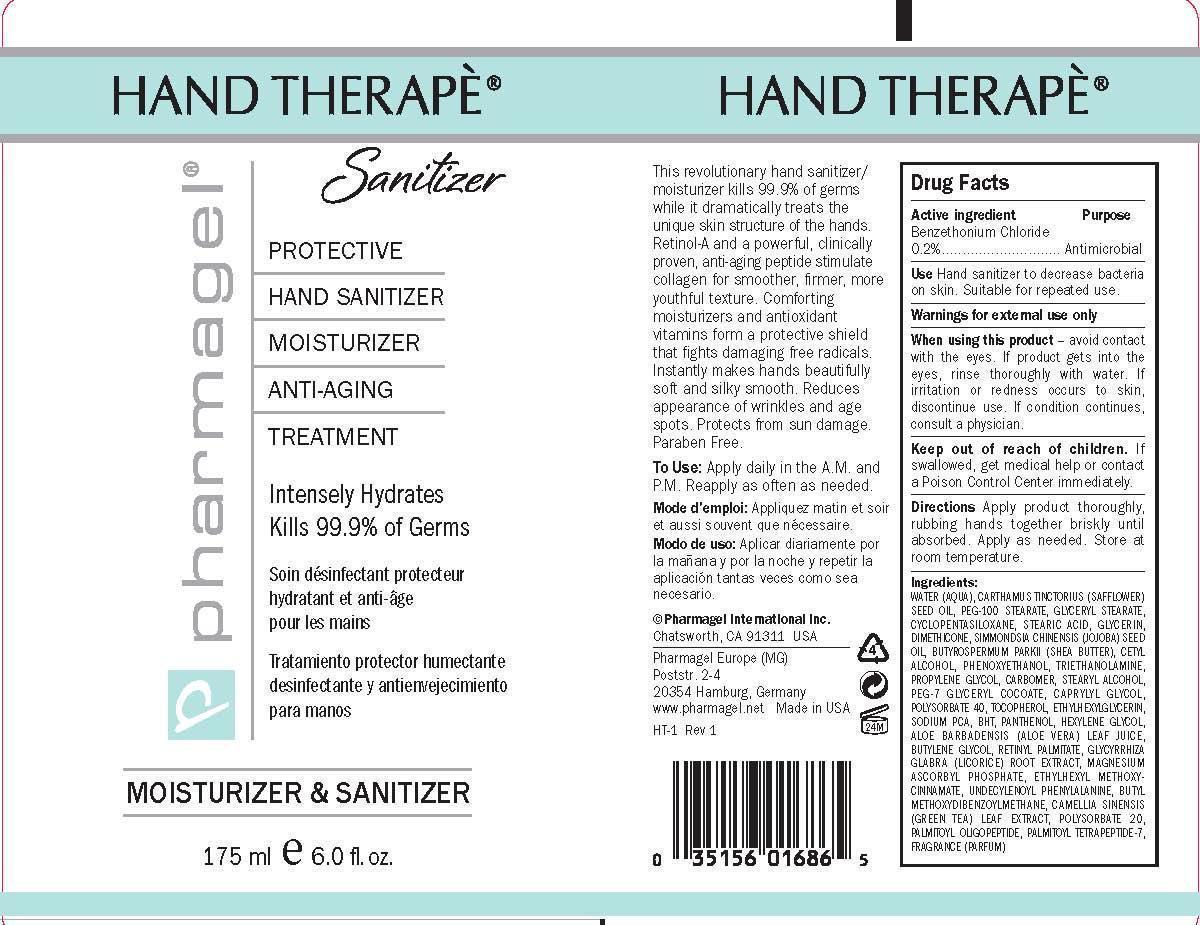
When using this product:
• avoid contact with the eyes. If product gets into the eyes, rinse thoroughly with water.
• if irritation or redness occurs to skin, discontinue use.
• if condition continues, consult a physician.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
DIRECTIONS
Apply product thoroughly, rubbing hands together briskly until absorbed. Apply as needed. Store at room temperature.
INACTIVE INGREDIENTS:
WATER (AQUA), CARTHAMUS TINCTORIUS (SAFFLOWER) SEED OIL, PEG-100 STEARATE, GLYCERYL STEARATE, CYCLOPENTASILOXANE, STEARIC ACID, GLYCERIN, DIMETHICONE, SIMMONDSIA CHINENSIS (JOJOBA) SEED OIL, BUTYROSPERMUM PARKII (SHEA BUTTER), CETYL ALCOHOL, PHENOXYETHANOL, TRIETHANOLAMINE, PROPYLENE GLYCOL, CARBOMER, STEARYL ALCOHOL, PEG-7 GLYCERYL COCOATE, CAPRYLYL GLYCOL, POLYSORBATE 40, TOCOPHEROL, ETHYLHEXYLGLYCERIN, SODIUM PCA, BHT, PANTHENOL, HEXYLENE GLYCOL, ALOE BARBADENSIS (ALOE VERA) LEAF JUICE, BUTYLENE GLYCOL, RETINYL PALMITATE, GLYCYRRHIZA GLABRA (LICORICE) ROOT EXTRACT, MAGNESIUM ASCORBYL PHOSPHATE, ETHYLHEXYL METHOXYCINNAMATE, UNDECYLENOYL PHENYLALANINE, BUTYL METHOXYDIBENZOYLMETHANE, CAMELLIA SINENSIS(GREEN TEA) LEAF EXTRACT, POLYSORBATE 20, PALMITOYL OLIGOPEPTIDE, PALMITOYL TETRAPEPTIDE-7, FRAGRANCE (PARFUM)